Hi Sam,
I am getting ready for the Coronavirus because the fools in Cheltenham UK are holding the Cheltenham Races which regularly brings in 250.000 people to my town, which will host one of the biggest Coronavirus incubation's in history when the punters hit the pubs and clubs. Many punters are coming from Ireland and abroad! This is all about money again! Anyway...
After reading the articles below, I am going to try to hit my gut and lung with both antivirals and antibiotics as well as using things like Quinine Sulphate that can be used for both Bacterial and Viral Infections, High Vit C, Zinc, Brewers Yeast (Saccharomyces cerevisiae, which is a fungus containing Virus,that is highly beneficial to the Gut and has the potential to destroy Clostridium Difficile and other dangerous Bacteria such as Streprococcus and seems to have Phage properties as Antiviral and Antibacterial) and I will possibly add part of a protocol for destroying Biofilms in D-Lacic acidosis and Bacterial Overgrowth (Interfase Plus, NAC and Serraptase) hopefully rotating these during the incubation period experimentally.
There may be some similarities with Coronavirus related SARS in that the body has to deal initially with two major infection sites in Gut and Lungs. The Virus needs host Bacteria to reproduce, so hitting Bacteria in the Gut and Lungs may help reduce infection by destroying possible hosts.
In Aids the Virus uses Gut Bacteria as a vehicle to break down the mucosal lining to cause permeability...... although diarrhea may not be caused by the Coronavirus, it may still cause Gastrointestinal symptoms where Gut Bacteria host the Coronavirus and compound the assault (I have often wondered if D-Lactic acidosis and other Bacterial Overgrowth's may be due not only to selection through Antibiotic Resistance but also possible Overgrowth due to Viral causes in reproducing within the Host Bacteria which usually remain as part of a balanced symbiosis).
Reports below;
1. Diarrhea is a rare symptom of COVID-19, but gastrointestinal symptoms like nausea and diarrhea could be early clues of infection, a
growing body of preliminary research has found. It's also a symptom mostly unique to people with COVID-19 and some children with the flu.
Still, the overlap between symptoms of COVID-19 and those of other common conditions is in part why widespread testing is necessary.
2. Crossing barriers: infections of the lung and the gut
Mucosal Immunology volume 2, pages100–102(2009)
Cite this article
Abstract
Although known as respiratory pathogens, severe acute respiratory syndrome (SARS) and its sister coronaviruses frequently cause enteric symptoms. In addition, other classically non-enteric viruses (such as HIV and influenza) may also have enteric effects that are crucial in their pathogeneses. These effects can be due to direct infection of the gut mucosa, but can also be because of decreased antibacterial defenses, increased mucosal permeability, bacterial translocation, and systemic leak of endotoxin.
Coronavirus Colds and Enteritis
Molecular detection methods show that picornaviruses (rhinovirus and enterovirus) cause approximately 60% of common colds in older children and adults. The next most common are the coronaviruses, causing about 15% of colds. Human coronaviruses are classified genetically into three groups. One of the group 2 viruses, OC43, shows remarkable antigenic and genetic similarities to a common bovine coronavirus that probably first mutated and transmitted to man in the 1890s.
1 Although now transmitted from person to person via the respiratory tract, OC43 causes gastrointestinal symptoms in up to 57% of infected people, along with various combinations of rhinitis (36.6%), pharyngitis (30%), and bronchitis or bronchiolitis (26.6%).
2 Therefore, gastrointestinal symptoms can be as prominent as respiratory symptoms in coronavirus colds, often labeled “gastric flu”
In veterinary practice, coronaviruses are also notorious for causing infection of either the gut or lung and for sometimes moving between sites. Porcine transmissible gastroenteritis virus (TGEV) is a coronavirus related to the 229E strain of coronavirus (another cause of common colds in man). TGEV was a major cause of severe gastroenteritis in domesticated pigs, causing significant morbidity and mortality throughout worldwide.
However, in 1984 spontaneous deletions caused a new strain to emerge transmitted via the respiratory route and causing predominately upper respiratory symptoms, and often mild or inapparent infection. This new virus was sufficiently antigenetically similar to TGEV to cause cross-protection,
3 so that the new strain virtually wiped out the parental strain. Therefore, the respiratory version of the coronavirus acted as a natural vaccine, eliminating TGEV as a significant veterinary problem.
SARS
When SARS broke out in the winter of 2002–2003, the world was gripped by a well-founded fear that it would become a lethal pandemic. The acronym resonated with the public, helping to focus attention not only on the virus but also on its transmissibility and global potential. The original animal reservoir of the SARS coronavirus appears to be wild bats, although it probably adapted to infect nocturnal pine civet cats before moving in to man.
4 Although SARS was dubbed “respiratory” the virus was clearly not just a lung pathogen—it also affected the gut.
The emerging epidemic came to the attention of virologists when it began to spread in Hong Kong in early March 2002. One of the first major outbreaks was in a hospital, triggered by a “superspreading” event following the admission of a doctor who had acquired the infection while working with patients with atypical pneumonia in Guangdong Province of mainland China. In all, 70 hospital staff became infected in this one outbreak; this same index case apparently infected visitors to the hotel where the doctor stayed, one of whom flew to Hanoi, was admitted to hospital and there led to an outbreak in which 63 hospital staff were infected. Events in the same hotel probably led to the transmission of infection to Toronto, where a major outbreak also occurred.
The major community transmission in Hong Kong occurred in a tower block complex named Amoy Gardens. In this case, the evidence points to transmission from soil pipes and sewage which appears to have led to aerosolization of SARS coronavirus, and to inhalation and transmission to approximately 331 new individuals. This alarming ability to swop between being a respiratory and a gastrointestinal pathogen was one of the features made SARS so potentially devastating.
SARS was characterized by intense systemic symptoms and triggering of exuberant host immune responses.
5 Detailed pathological studies showed that SARS coronavirus could infect not only the respiratory epithelium, but also surface enterocytes in the small bowel. Although infection caused diffuse alveolar damage, the changes in the gut are more subtle
6 and might include an increase in intestinal permeability to lipopolysaccharide (LPS) and transmigration of intestinal bacteria (
Figure 1c).
Figure 1
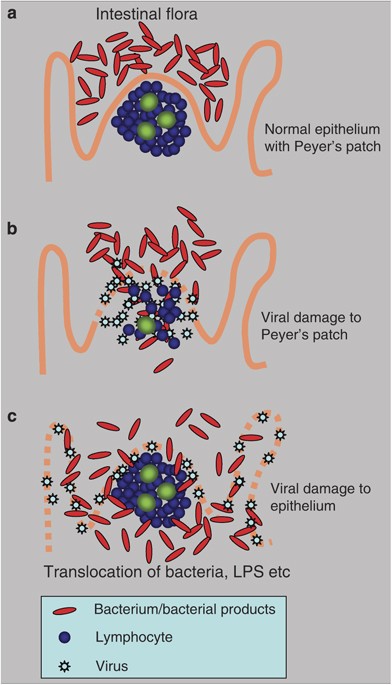
Alternative mechanisms of mucosal viral–bacterial interactions. (
a) In the normal gut, intestinal flora are kept from invasion by the intact mucosa, even in the follicle-associated epithelium that specializes in transportation of antigen into the Peyer's patch. (
b) If the submucosal lymphoid tissue is damaged by infection (e.g., with HIV
11), the mucosa becomes permeable to bacteria and to bacterial products. Translocation of bacteria and bacterial products from the intestinal lumen cause systemic innate stimulation, leading to malaise and other systemic symptoms. (
c) If a virus is tropic for the intestinal epithelial cells, it causes cell damage and loosens the normally impermeable barrier that keeps bacteria in the intestinal lumen.
PowerPoint slide
Full size image
Remarkably, the onset of diarrhea in SARS infected patients usually peaked on days 4–9, by which time the fever had subsided. However, the coronavirus copy number in some studies showed an increase between day 5 and day 10, so that maximal infectivity followed the fever,
7 leading perhaps to a false sense of security amongst those caring for SARS patients. Although virus copy number in the pharyngeal aspirates dropped significantly between days 10 and 15, many patients still had SARS coronavirus present in the stool at day 21, by which time under 50% had virus present in the nasopharyngeal aspirate. Therefore, late transmission by contact with stool was a particular unappreciated risk.
Interactions Between Viruses and Bacteria
Infection with porcine respiratory coronavirus or porcine reproductive and respiratory syndrome virus has a synergistic effect with bacterial products, demonstrated by enhanced inflammation induced by LPS from
Escherichia coli. LPS caused potentiation of disease, with enhanced production of tumor necrosis factor, interleukin-1, and interleukin-6.
8
It has been known for many years that healthy people often carry pathogenic bacteria (such as
Neisseria meningitides or
Streptococcus pneumoniae) in the upper respiratory tract, but may only develop invasive disease during coinfection with respiratory viruses. The reason for these interactions are incompletely understood, but intriguing recent study show that influenza and respiratory syndrome virus are both capable of causing a persistent inhibition of the innate response to bacterial superinfection, and therefore to increased bacterial replication and disease.
9
Intestinal Effects of HIV Infection
Although dual tropism for both the lung and gut may be an obvious and clear reason to consider the gut in respiratory infection, links can also be more subtle and complex. HIV targets CD4-expressing T cells and macrophages, thereby leading to immunosuppression. However, the symptoms of progressive HIV infection sometimes include profound weight loss (“slim disease”), and evidence of systemic immune activation. Indeed, this immune activation may assist fresh infection by HIV of activated T cells, so enhancing the viral life cycle.
The reasons for this immune activation seems to include the fact that HIV infects intestinal mucosal lymphocytes, including those in the Peyer's patch and especially Th17 cells that normally keep bacteria in check
10 (
Figure 1b). This leads to enhanced gastrointestinal permeability to microbial products, causing increased levels of circulating LPS,
11 further activating the innate and adaptive immune system. An additional intriguing possibility is that the distribution of FoxP3+ regulatory T cells is affected by HIV infection,
12 and that the balance between proinflammatory and antiviral effects is disturbed
13 thereby contributing to immune activation, malaise, and cachexia in HIV-infected patients.
Intestinal Infection by Influenza
Highly pathogenic strains of influenza also cause intense systemic symptoms, sometimes associated with gastrointestinal disease. In waterfowl, influenza is mostly an enteric pathogen, transmitted via the feces in the lakes and waterways, and efficiently transmitted to other birds feeding and breeding on the same water. When these viruses spread to man, viral replication can trigger hypercytokinaemia.
14
It therefore seems possible that the gut (known to be susceptible to infection with highly pathogenic strains of influenza)
15 might also become permeable to intestinal LPS in severe influenza infections (
Figure 1c), or that bacterial translocation through the gut wall could contribute to systemic symptoms, cytokine release and circulatory collapse.
Conclusions
In the era of integrative systems biology and holistic medicine, it is not only timely but also essential to view the gut and the lung as a continuous surface with distinct but overlapping susceptibilities. In addition, viral and bacterial infections should not be regarded as isolated events but viewed both in the context of intercurrent infection and of previous infection history. Studies of single infections are certainly revealing, but in reality the secret to understanding variations in responses to infections must include an awareness of what else is present on a rich polymicrobial landscape.
Brewer's yeast is a kind of yeast that is a by-product of brewing beer.
Dietary supplements containing brewer's yeast often contain non-living, dried yeast. People use brewer's yeast to make medicine.
Brewer's yeast is taken by
mouth for respiratory problems, including the
common cold and other upper respiratory tract infections, influenza,
seasonal allergies, and
swine flu. Brewer's yeast is also taken by
mouth for
diarrhea, swelling of the
colon (
colitis) due to the bacteria
Clostridium difficile,
high cholesterol, loss of appetite,
acne, premenstrual syndrome (PMS), recurring boils on the
skin (furunculosis),
type 2 diabetes and
irritable bowel syndrome (
IBS). It has also been used as a source of B
vitamins,
chromium, and protein.
How does it work?
Due to the chromium content of brewer's yeast, there is interest in using it for lowering blood glucose in people with diabetes. Chromium may help the body use insulin more effectively. This can lower blood sugar levels.
Additionally, brewer's yeast seems to increase enzymes in the intestine that could help relieve diarrhea.
Brewer's yeast might help fight bacteria that cause infections in the intestine and improve the body's defenses against viral lung infections such as flu and the common cold.







