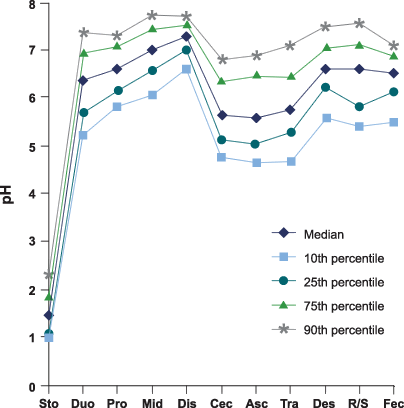
Actually the RPS [Raw Potato Starch] studies are that [RS2] rapidly ferments in the caecum, but not much elsewhere. Stool pH does not change much. RPS fails to dilute ammonia or other fecal carcinogens.
When however insoluble fiber (5% cellulose, that's quite a bit for human diets -- 20 g/day, ~4 Tbs psyllium rough equivalent), then fermentation appears to be carried out throughout the entire GIT. pH lowers significantly and in the stool. Ammonia is diluted, etc
I've read about alkalinity and SIFO but I don't have a reference. The lack of commensals in the SI is what allows the candida to overbreed there where the environment is nutrient/carb rich.
When lactobacilli and other commensals are re-established, studies show that candida is either prevented or can be reduced. However once candida is colonized and firmly established (like it likes to do, especially when mercury burdens are high), very hard to fix the SI I've seen and found.
Lp, Enterococcus (probiotic), SBOs etc are all antifungal too.
Antibiotics and parasites are the triggers candida, then subsequent autoimmune diseases/CFS/etc. I'm finding this connection everywhere. Here's the latest on both autism and celiac and early use of antibiotics. The problem is nearly every mom has had antibiotics or microbes are lost thru disease, gastroenteritis, infection or diet.
Not everyone has celiac, but the epidemic of gluten sensitivity derives from common root causes when the SI degrades and can't perform its functions.
http://physrev.physiology.org/content/physrev/90/3/859.full.pdf
Autism Very little is known about the underlying etiology of autism. Extensive antibiotic use is commonly associated with late-onset autism (18–24 mo of age), causing some to hypothesize that disruptions in the normal microbiota may allow colonization by autism-triggering microorganism( s), or promote the overgrowth of neurotoxin-producing bacteria like Clostridium tetani (24). The link between the intestinal microbiota and autism is supported by the following observations: 1) disease onset often follows antimicrobial therapy, 2) gastrointestinal abnormalities are often present at the onset of autism and frequently persist, and 3) autistic symptoms have sometimes been reduced by oral vancomycin treatment, while relapse occurs following cessation of treatment (71, 259). These observations have been supported by qPCR (279) and culture-based (71) microbiota profiling techniques, which indicate that certain clusters of Clostridium spp. are present at 10-fold higher numbers in stool samples from autistic children compared with healthy controls.
Furthermore, the authors suggest that it is not a mere coincidence that exposure to trimethoprim/sulfamethoxazole antibiotics is much more likely to precede diagnosis of late-onset autism than exposure to any other antibiotic regimen. Trimethoprim/sulfamethoxazole antibiotics are not effective against Clostridium spp., suggesting that early exposure to these drugs may promote an overgrowth of Clostridium spp. that could contribute to the etiology of autism (71). Interestingly, oral vancomycin specifically targets Gm organisms, among them Clostridium spp. Clostridia spores that remain viable after vancomycin treatment are believed to be responsible for the relapses that occur in autistic patients after discontinuation of vancomycin. One group has even suggested that Clostridia spores are the reason why high rates of autism are seen among siblings (70). Finegold et al. (71) suggested a number of mechanisms whereby the gut microbiota could be responsible for the debilitation of regressive autism including neurotoxin production by a subset of abnormal flora, autoantibody production that results in the attack on neuron-associated proteins, or microbial production of toxic metabolites that have neurological side effects (71).
Am J Epidemiol. 2014 May 22. pii: kwu101. [Epub ahead of print]
Association of Maternal Education, Early Infections, and Antibiotic Use With Celiac Disease: A Population-Based Birth Cohort Study in Northeastern Italy.
Canova C,
Zabeo V,
Pitter G,
Romor P,
Baldovin T,
Zanotti R,
Simonato L.
Abstract
We conducted a population-based birth cohort study of approximately 203,000 babies born in northeastern Italy (1989-2012) to investigate perinatal variables, early infections leading to hospital admission, and antibiotic use in the first 12 months of life as possible risk factors for celiac disease (CD). Incident CD cases were identified from pathology reports, hospital discharge records, and exemptions from prescription charges for clinical tests. Multivariate Poisson regression models were fitted to estimate incidence rate ratios (IRRs). A total of 1,227 children had CD; CD was histopathologically confirmed in 866 (71%). Female sex, maternal age, and high maternal educational level were found to be significantly associated with CD. Gastrointestinal infections were strongly associated with a subsequent diagnosis of CD (IRR = 2.04, 95% confidence interval (CI): 1.30, 3.22). Antibiotic use was significantly associated with CD onset (IRR = 1.24, 95% CI: 1.07, 1.43), with a dose-response relationship for number of courses (P-trend < 0.01). Cephalosporin use strongly increased the risk of CD (IRR = 1.42, 95% CI: 1.18, 1.73). Use of antibiotics (supported by the dose-response relationship) and gastrointestinal infections in the first year of life may facilitate the early onset of CD by altering intestinal microflora and the gut mucosal barrier. Perinatal factors, including cesarean section, had little influence on the risk of childhood CD.
BMC Gastroenterol. 2013 Jul 8;13:109. doi: 10.1186/1471-230X-13-109.
Antibiotic exposure and the development of coeliac disease: a nationwide case-control study.
Mårild K1,
Ye W,
Lebwohl B,
Green PH,
Blaser MJ,
Card T,
Ludvigsson JF.
Author information
Abstract
BACKGROUND:
The intestinal microbiota has been proposed to play a pathogenic role in coeliac disease (CD). Although antibiotics are common environmental factors with a profound impact on intestinal microbiota, data on antibiotic use as a risk factor for subsequent CD development are scarce.
METHODS:
In this population-based case-control study we linked nationwide histopathology data on 2,933 individuals with CD (Marsh stage 3; villous atrophy) to the Swedish Prescribed Drug Register to examine the association between use of systemic antibiotics and subsequent CD. We also examined the association between antibiotic use in 2,118 individuals with inflammation (Marsh 1-2) and in 620 individuals with normal mucosa (Marsh 0) but positive CD serology. All individuals undergoing biopsy were matched for age and sex with 28,262 controls from the population.
RESULTS:
Antibiotic use was associated with CD (Odds ratio [OR] = 1.40; 95% confidence interval [CI] = 1.27-1.53), inflammation (OR = 1.90; 95% CI = 1.72-2.10) and normal mucosa with positive CD serology (OR = 1.58; 95% CI = 1.30-1.92). ORs for prior antibiotic use in CD were similar when we excluded antibiotic use in the last year (OR = 1.30; 95% CI = 1.08-1.56) or restricted to individuals without comorbidity (OR = 1.30; 95% CI = 1.16 - 1.46).
CONCLUSIONS:
The positive association between antibiotic use and subsequent CD but also with lesions that may represent early CD suggests that intestinal dysbiosis may play a role in the pathogenesis of CD. However, non-causal explanations for this positive association cannot be excluded.
Nutr Hosp. 2013 Mar-Apr;28(2):464-73. doi: 10.3305/nh.2013.28.2.6310.
Influence of early environmental factors on lymphocyte subsets and gut microbiota in infants at risk of celiacdisease; the PROFICEL study.
Pozo-Rubio T1, de Palma G, Mujico JR, Olivares M, Marcos A, Acuña MD, Polanco I, Sanz Y, Nova E.
Author information
Abstract
in
English,
Spanish
INTRODUCTION:
It is known that the HLA genotype can explain about a 40% of the genetic risk of celiac disease (CD), thus, other genetic predisposing factors as well as factors that subtly modulate T cell activation and differentiation need to be studied. This includes environmental factors that are currently believed to impact on the immune system and gut microbiota development.
AIM:
To assess the associations between early environmental factors (EEF), lymphocyte subsets, and intestinal microbiota composition in infants at familial risk for CD.
STUDY DESIGN:
Prospective observational study.
SUBJECTS:
Fifty-five 4 month-old infants with at least a first-degree relative suffering CD. Infants were classified according to type of delivery, mother's antibiotic intake during pregnancy and during labor, milk-feeding practices, early infections and antibiotic intake, rotavirus vaccine administration, and allergy incidence within the first 18 months of life.
METHODS:
Lymphocyte subsets and gut microbiota composition were studied at the age of 4 months.
RESULTS:
Formula feeding and infant's infections were associated with higher CD3+, CD4+, CD4+CD38+, CD4+CD28+ and CD3+CD4+CD45RO+ counts (P0.01). Infant s infections were also associated with higher CD4+CD25+, CD4+HLA-DR+ and NK cell counts (P0.01). Cesarean delivery and rotavirus vaccine administration were associated with lower percentage of CD4+CD25+ cells. Infant's antibioticintake was associated and correlated with lower counts of Bifidobacterium longum and higher counts of Bacteroides fragilis group.
CONCLUSIONS:
Infant s infections and antibiotic intake in the first 4 months of life are the EEF more strongly and/or frequently associated to lymphocyte subpopulations and microbiota composition, respectively, in infants at risk of CD.