nerd
Senior Member
- Messages
- 863
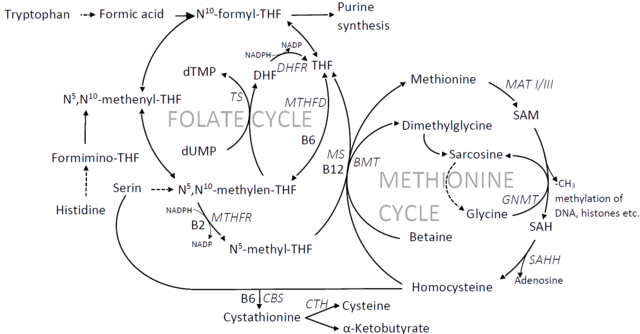
I've read different viewpoints of what the problem is with methylation and its potential causes. I have my own theory about this, but I don't think I'm aware of all the existing theories so that I can check if it is consistent with all the evidence that is linked to the methylation cycle. This is why I'd like to reopen the discussion about the methylation cycle. I'd ask for your opinions about what is going wrong and the different states that the trapped methylation cycle has. I'd appreciate references to any research that support these theories and to potential causally related findings. Maybe we can have an updated compendium here of all the possibilities and their consistencies and inconsistencies with research. Let's narrow this down step by step as far as we can.
Thanks in advance!
@Pyrrhus @drmullin30 @mitoMAN
Thanks in advance!

@Pyrrhus @drmullin30 @mitoMAN
