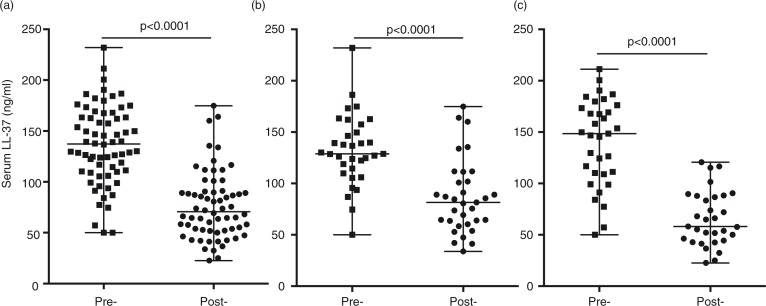
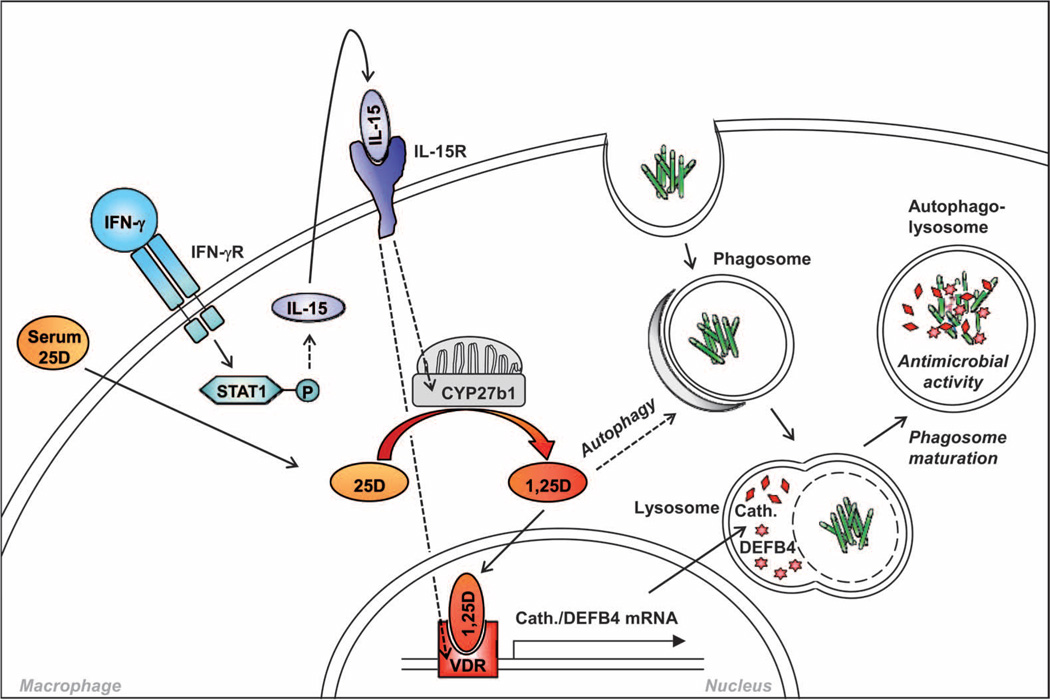
uglevod
Senior Member
- Messages
- 220
[2020] Higher 25-hydroxyvitamin D level is associated with increased risk for Behçet's disease
https://pubmed.ncbi.nlm.nih.gov/32593521/
Background & aim: Previous studies showed a vitamin D deficiency in patients with Behçet's disease, suggesting potential benefits of vitamin D supplementation in the prevention and treatment of Behçet's disease. Interpretation of these studies may be limited by reverse causality or confounding bias. We aim to determine the causal association between serum 25-hydroxyvitamin D [25(OH)D] and the risk of Behçet's disease by Mendelian randomization.
On the basis of evidence in 7909 human beings, this study provides the newest indication that a lifelong higher 25(OH)D level is associated with an increased risk of Behçet's disease. Special attention should be paid to the potential harm of long-term or high-dose use of vitamin D supplements in clinical practice.
follow-up to:
[2014] Vitamin D deficiency in patients with Behcet’s disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3996194/
[2017] High Vitamin D Levels May Downregulate Inflammation in Patients with Behçet's Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474237/
[2011] Vitamin D status in patients with Behcet's Disease
https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322011000500002
In patients with Behcet's Disease, 25-hydroxyvitamin D values were significantly lower than those of the healthy controls (p<0.001). Serum Ca, P, and ALP levels were similar in both groups. Serum ESR and CRP levels were significantly higher in patients than controls (p<0.05). There was no correlation between 25-hydroxyvitamin D levels and age, body mass index (BMI), disease duration, ESR, or CRP levels.
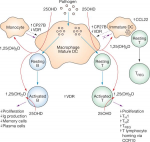
[2008] Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet's disease
https://pubmed.ncbi.nlm.nih.gov/18411217/
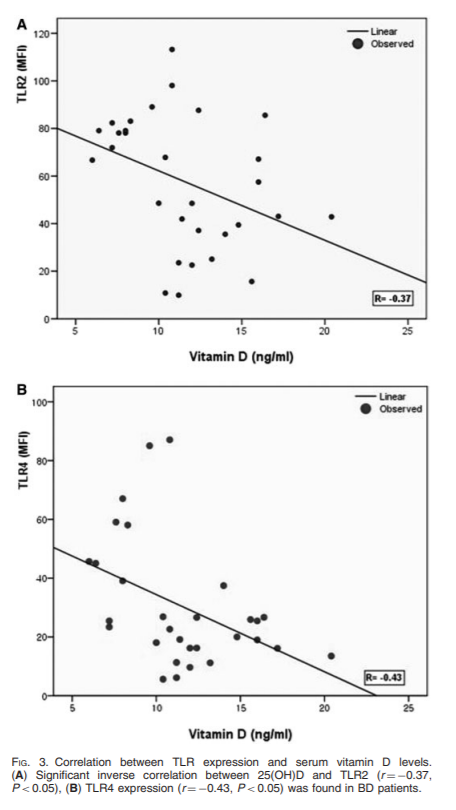
Objectives: Recent studies have shown the immunomodulatory effect of vitamin D(3) through down-regulation of Toll-like receptor (TLR) expression in human monocytes. To understand the implication of innate immunity with the role of vitamin D affecting TLR expression in Behçet's disease (BD), we focused on the association between the TLR expression and the serum vitamin D concentration in BD.
Methods: The expression of TLR2, TLR4 and CD16 on monocytes was detected by flow cytometric analysis and RT-PCR. Serum 25-hydroxyvitamin D [25(OH)D] levels were measured in the patients with BD, psoriasis and healthy controls, and then the expression of TLRs was correlated with the value of serum 25(OH)D levels. To assess the influence of vitamin D(3) on expression and function of TLRs in vitro, human monocytes were treated with increasing concentrations of 1,25(OH)(2)D(3).
Results: We found that the monocytes of active BD patients showed higher expressions of TLR2 and TLR4 than those of controls, and serum 25(OH)D levels tended to be lower in active BD. Furthermore, 25(OH)D levels were inversely correlated with the expressions of TLR2, TLR4 and clinical indicators. In vitro analysis showed that vitamin D(3) was found to dose-dependently suppress the protein and mRNA expressions of TLR2 and TLR4. TNF-alpha synthesis was also decreased upon TLR ligand stimulation in vitamin D(3)-treated monocytes.
Conclusion: These results suggest that the inflammation triggered through TLR2 and TLR4 is important in the pathogenesis of BD. And it seems possible that vitamin D may be used as a therapeutic option by modulating TLR2 and TLR4 expression of monocytes in BD.
https://pubmed.ncbi.nlm.nih.gov/10549626/
Toll-like receptor (TLR) 2 and TLR4 are implicated in the recognition of various bacterial cell wall components, such as lipopolysaccharide (LPS). To investigate in vivo roles of TLR2, we generated TLR2-deficient mice. In contrast to LPS unresponsiveness in TLR4-deficient mice, TLR2-deficient mice responded to LPS to the same extent as wild-type mice. TLR2-deficient macrophages were hyporesponsive to several Gram-positive bacterial cell walls as well as Staphylococcus aureus peptidoglycan. TLR4-deficient macrophages lacked the response to Gram-positive lipoteichoic acids. These results demonstrate that TLR2 and TLR4 recognize different bacterial cell wall components in vivo and TLR2 plays a major role in Gram-positive bacterial recognition.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1007390
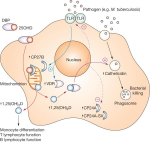
What is TLR4 and what are its ligands?
The innate immune system recognizes pathogen-associated molecular patterns (PAMPs) of viral or bacterial intruders via pattern recognition receptors (PRRs). This includes the family of TLRs that consists of related, transmembrane proteins that play a central role in the initiation of inflammatory responses, including the secretion of cytokines and chemokines.
TLR4, which is mainly expressed on cells of the immune system—including monocytes, macrophages and dendritic cells—has long been recognized as a PRR that senses lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria. Activation of TLR4 by LPS, its best studied ligand, is a multistep process. The initial step involves the LPS binding protein (LBP) which extracts LPS from bacterial membranes and LPS-containing vesicles to transfer it to the TLR4 coreceptor cluster of differentiation 14 (CD14). CD14 exists in two forms, soluble and membrane-bound. Both forms are able to interact with LPS-loaded LBP. CD14 breaks down LPS aggregates and transfers monomeric LPS into a hydrophobic pocket on myeloid differentiation factor 2 (MD-2) that is part of the MD-2/TLR4 complex. The high-affinity binding of LPS leads to dimerization and activation of the MD-2/TLR4 complex [6, 7]. Activation of TLR4 results in the recruitment of the intracellular adaptor protein, myeloid differentiation primary response 88 (MyD88), and/or toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF), ultimately resulting in the expression and secretion of pro-inflammatory mediators [6, 7].
TLR4 has also been shown to be a sensor for damage-associated molecular patterns (DAMPs). These include a wide variety of molecules released from injured or dying tissues as well as molecules actively released in response to cellular stress from intact cells [6, 8]. In addition to bacterial PAMPs and cellular DAMPs, TLR4 also recognizes PAMPs from other pathogens including fungi, parasites, and viruses [9]. How the TLR4 complex is activated by DAMPs and non-LPS PAMPs, which vary widely in their structure—some with no structural similarities to LPS [8, 10]—remains to be determined. Resolving the structure of these complexes is a critical part toward dissecting their mechanisms of activation.
___
So called "Behcet’s disease" is another good example of basket-case diagnosis of a chronic, body-wide inflammation:
https://www.mayoclinic.org/diseases-conditions/behcets-disease/diagnosis-treatment/drc-20351331
If you have moderate to severe Behcet's disease, your doctor might prescribe: Corticosteroids to control inflammation. Corticosteroids, such as prednisone, are used reduce the inflammation caused by Behcet's disease. Doctors often prescribe them with another medication to suppress the activity of your immune system.
Scientists discover how neuroactive steroids dampen inflammatory signaling in cells
https://www.sciencedaily.com/releases/2019/02/190213142713.htm
For the first time, scientists discovered how neuroactive steroids naturally found in the brain and bloodstream inhibit the activity of a specific kind of protein called Toll-like receptors (TLR4), which have been known to play a role in inflammation in many organs, including the brain.
The effect of systemic corticosteroid therapy on the expression of toll-like receptor 2 and toll-like receptor 4 in the cutaneous lesions of leprosy Type 1 reactions
https://pubmed.ncbi.nlm.nih.gov/22348338/
We have demonstrated that the gene hARP-P0 is a suitable control gene for TLR gene expression studies in this population. The gene and protein expression of TLR2 and TLR4 were both reduced significantly during corticosteroid treatment.
____
Potential Infectious Etiology of Behçet's Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255303/
Behçet's disease is a multisystem inflammatory disorder characterized by recurrent oral aphthous ulcers, genital ulcers, uveitis, and skin lesions. The cause of Behçet's disease remains unknown, but epidemiologic findings suggest that an autoimmune process is triggered by an environmental agent in a genetically predisposed individual. An infectious agent could operate through molecular mimicry, and subsequently the disease could be perpetuated by an abnormal immune response to an autoantigen in the absence of ongoing infection. Potential bacteria are Saccharomyces cerevisiae, mycobacteria, Borrelia burgdorferi, Helicobacter pylori, Escherichia coli, Staphylococcus aureus, and Mycoplasma fermentans, but the most commonly investigated microorganism is Streptococcus sanguinis. The relationship between streptococcal infections and Behçet's disease is suggested by clinical observations that an unhygienic oral condition is frequently noted in the oral cavity of Behçet's disease patients. Several viral agents, including herpes simplex virus-1, hepatitis C virus, parvovirus B19, cytomegalovirus, Epstein-Barr virus and varicella zoster virus, may also have some role.
https://pubmed.ncbi.nlm.nih.gov/32593521/
Background & aim: Previous studies showed a vitamin D deficiency in patients with Behçet's disease, suggesting potential benefits of vitamin D supplementation in the prevention and treatment of Behçet's disease. Interpretation of these studies may be limited by reverse causality or confounding bias. We aim to determine the causal association between serum 25-hydroxyvitamin D [25(OH)D] and the risk of Behçet's disease by Mendelian randomization.
On the basis of evidence in 7909 human beings, this study provides the newest indication that a lifelong higher 25(OH)D level is associated with an increased risk of Behçet's disease. Special attention should be paid to the potential harm of long-term or high-dose use of vitamin D supplements in clinical practice.
follow-up to:
[2014] Vitamin D deficiency in patients with Behcet’s disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3996194/
[2017] High Vitamin D Levels May Downregulate Inflammation in Patients with Behçet's Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474237/
[2011] Vitamin D status in patients with Behcet's Disease
https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322011000500002
In patients with Behcet's Disease, 25-hydroxyvitamin D values were significantly lower than those of the healthy controls (p<0.001). Serum Ca, P, and ALP levels were similar in both groups. Serum ESR and CRP levels were significantly higher in patients than controls (p<0.05). There was no correlation between 25-hydroxyvitamin D levels and age, body mass index (BMI), disease duration, ESR, or CRP levels.
[2008] Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet's disease
https://pubmed.ncbi.nlm.nih.gov/18411217/
Objectives: Recent studies have shown the immunomodulatory effect of vitamin D(3) through down-regulation of Toll-like receptor (TLR) expression in human monocytes. To understand the implication of innate immunity with the role of vitamin D affecting TLR expression in Behçet's disease (BD), we focused on the association between the TLR expression and the serum vitamin D concentration in BD.
Methods: The expression of TLR2, TLR4 and CD16 on monocytes was detected by flow cytometric analysis and RT-PCR. Serum 25-hydroxyvitamin D [25(OH)D] levels were measured in the patients with BD, psoriasis and healthy controls, and then the expression of TLRs was correlated with the value of serum 25(OH)D levels. To assess the influence of vitamin D(3) on expression and function of TLRs in vitro, human monocytes were treated with increasing concentrations of 1,25(OH)(2)D(3).
Results: We found that the monocytes of active BD patients showed higher expressions of TLR2 and TLR4 than those of controls, and serum 25(OH)D levels tended to be lower in active BD. Furthermore, 25(OH)D levels were inversely correlated with the expressions of TLR2, TLR4 and clinical indicators. In vitro analysis showed that vitamin D(3) was found to dose-dependently suppress the protein and mRNA expressions of TLR2 and TLR4. TNF-alpha synthesis was also decreased upon TLR ligand stimulation in vitamin D(3)-treated monocytes.
Conclusion: These results suggest that the inflammation triggered through TLR2 and TLR4 is important in the pathogenesis of BD. And it seems possible that vitamin D may be used as a therapeutic option by modulating TLR2 and TLR4 expression of monocytes in BD.

https://pubmed.ncbi.nlm.nih.gov/10549626/
Toll-like receptor (TLR) 2 and TLR4 are implicated in the recognition of various bacterial cell wall components, such as lipopolysaccharide (LPS). To investigate in vivo roles of TLR2, we generated TLR2-deficient mice. In contrast to LPS unresponsiveness in TLR4-deficient mice, TLR2-deficient mice responded to LPS to the same extent as wild-type mice. TLR2-deficient macrophages were hyporesponsive to several Gram-positive bacterial cell walls as well as Staphylococcus aureus peptidoglycan. TLR4-deficient macrophages lacked the response to Gram-positive lipoteichoic acids. These results demonstrate that TLR2 and TLR4 recognize different bacterial cell wall components in vivo and TLR2 plays a major role in Gram-positive bacterial recognition.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1007390
What is TLR4 and what are its ligands?
The innate immune system recognizes pathogen-associated molecular patterns (PAMPs) of viral or bacterial intruders via pattern recognition receptors (PRRs). This includes the family of TLRs that consists of related, transmembrane proteins that play a central role in the initiation of inflammatory responses, including the secretion of cytokines and chemokines.
TLR4, which is mainly expressed on cells of the immune system—including monocytes, macrophages and dendritic cells—has long been recognized as a PRR that senses lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria. Activation of TLR4 by LPS, its best studied ligand, is a multistep process. The initial step involves the LPS binding protein (LBP) which extracts LPS from bacterial membranes and LPS-containing vesicles to transfer it to the TLR4 coreceptor cluster of differentiation 14 (CD14). CD14 exists in two forms, soluble and membrane-bound. Both forms are able to interact with LPS-loaded LBP. CD14 breaks down LPS aggregates and transfers monomeric LPS into a hydrophobic pocket on myeloid differentiation factor 2 (MD-2) that is part of the MD-2/TLR4 complex. The high-affinity binding of LPS leads to dimerization and activation of the MD-2/TLR4 complex [6, 7]. Activation of TLR4 results in the recruitment of the intracellular adaptor protein, myeloid differentiation primary response 88 (MyD88), and/or toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF), ultimately resulting in the expression and secretion of pro-inflammatory mediators [6, 7].
TLR4 has also been shown to be a sensor for damage-associated molecular patterns (DAMPs). These include a wide variety of molecules released from injured or dying tissues as well as molecules actively released in response to cellular stress from intact cells [6, 8]. In addition to bacterial PAMPs and cellular DAMPs, TLR4 also recognizes PAMPs from other pathogens including fungi, parasites, and viruses [9]. How the TLR4 complex is activated by DAMPs and non-LPS PAMPs, which vary widely in their structure—some with no structural similarities to LPS [8, 10]—remains to be determined. Resolving the structure of these complexes is a critical part toward dissecting their mechanisms of activation.
___
So called "Behcet’s disease" is another good example of basket-case diagnosis of a chronic, body-wide inflammation:
https://www.mayoclinic.org/diseases-conditions/behcets-disease/diagnosis-treatment/drc-20351331
If you have moderate to severe Behcet's disease, your doctor might prescribe: Corticosteroids to control inflammation. Corticosteroids, such as prednisone, are used reduce the inflammation caused by Behcet's disease. Doctors often prescribe them with another medication to suppress the activity of your immune system.
Scientists discover how neuroactive steroids dampen inflammatory signaling in cells
https://www.sciencedaily.com/releases/2019/02/190213142713.htm
For the first time, scientists discovered how neuroactive steroids naturally found in the brain and bloodstream inhibit the activity of a specific kind of protein called Toll-like receptors (TLR4), which have been known to play a role in inflammation in many organs, including the brain.
The effect of systemic corticosteroid therapy on the expression of toll-like receptor 2 and toll-like receptor 4 in the cutaneous lesions of leprosy Type 1 reactions
https://pubmed.ncbi.nlm.nih.gov/22348338/
We have demonstrated that the gene hARP-P0 is a suitable control gene for TLR gene expression studies in this population. The gene and protein expression of TLR2 and TLR4 were both reduced significantly during corticosteroid treatment.
____
Potential Infectious Etiology of Behçet's Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255303/
Behçet's disease is a multisystem inflammatory disorder characterized by recurrent oral aphthous ulcers, genital ulcers, uveitis, and skin lesions. The cause of Behçet's disease remains unknown, but epidemiologic findings suggest that an autoimmune process is triggered by an environmental agent in a genetically predisposed individual. An infectious agent could operate through molecular mimicry, and subsequently the disease could be perpetuated by an abnormal immune response to an autoantigen in the absence of ongoing infection. Potential bacteria are Saccharomyces cerevisiae, mycobacteria, Borrelia burgdorferi, Helicobacter pylori, Escherichia coli, Staphylococcus aureus, and Mycoplasma fermentans, but the most commonly investigated microorganism is Streptococcus sanguinis. The relationship between streptococcal infections and Behçet's disease is suggested by clinical observations that an unhygienic oral condition is frequently noted in the oral cavity of Behçet's disease patients. Several viral agents, including herpes simplex virus-1, hepatitis C virus, parvovirus B19, cytomegalovirus, Epstein-Barr virus and varicella zoster virus, may also have some role.
Last edited: