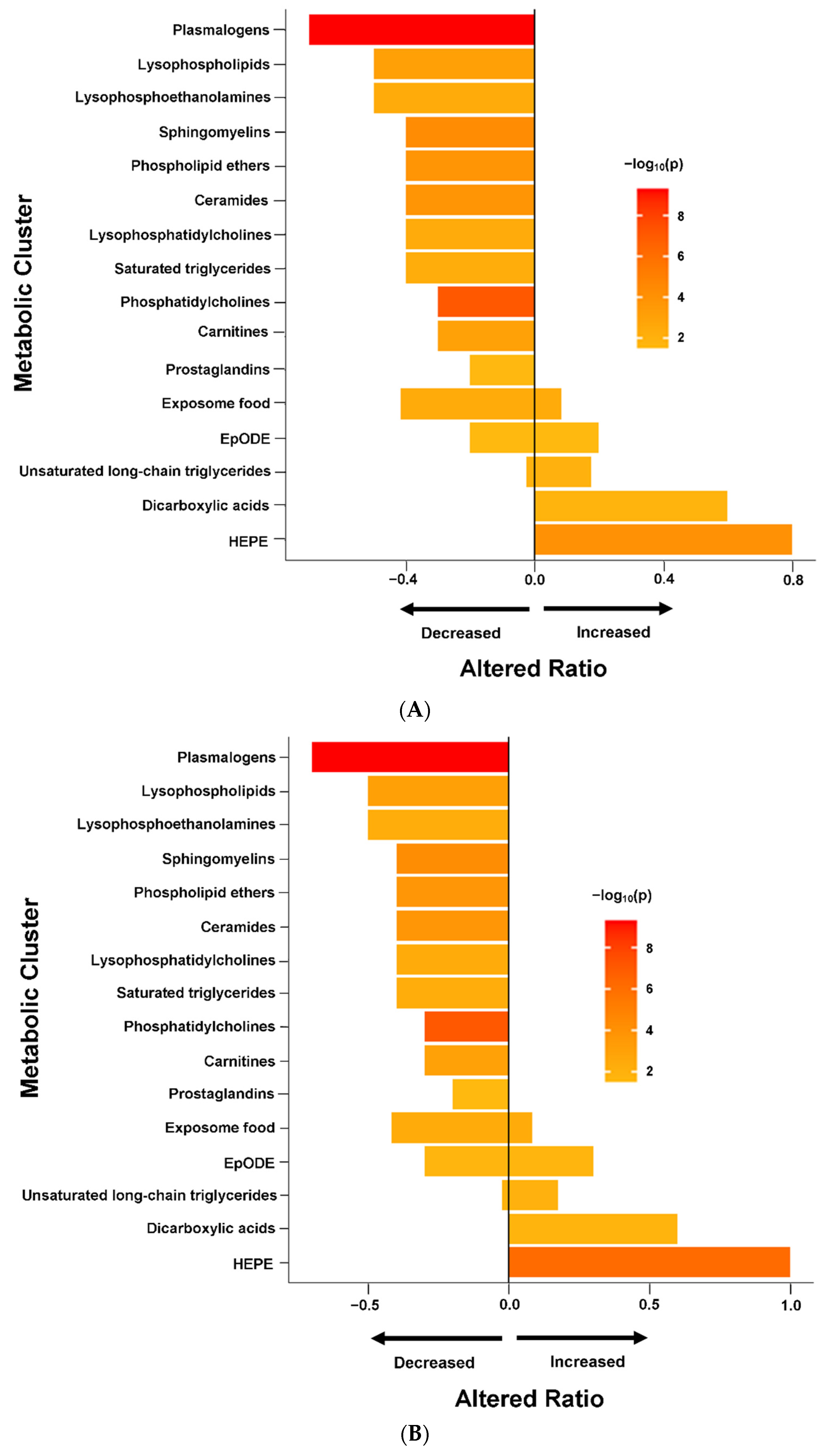
True. From the quote below i thought they put the whole blame for mecfs on the crosstalk in particular but they dont actually do that.I would think that it is indeed relevant for neurons in the brain. Yes, neurons don't utilize lipids for energy as much as other cells do. But neurons do engage in a lot of lipid metabolism for non-energy reasons, especially in order to maintain their dynamic membrane components, which are essential for the specific functions of neurons.
We posit that this crosstalk between mitochondria and peroxisomes plays an important role in maintaining energy homeostasis, and that dysregulation contributes to the fatigue and cognitive dysfunction that are hallmarks of ME/CFS
Last edited: