Major Study Links Gut Bacteria to Fatigue in Chronic Fatigue Syndrome (ME/CFS)
by
Cort Johnson | Nov 10, 2021 |
Gut,
Homepage,
Microbiome |
87 comments
Emailed to me By SamB;
Led by Cheng Guo (lead author) and Brent L. Williams (senior author) this big NIH-funded study from Ian Lipkin’s Columbia team at the “
Center for Solutions for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (CfS for ME/CFS)” set out to fix some of the problems found in prior gut studies. The study “
Deficient butyrate-producing capacity in the gut microbiome of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients is associated with fatigue symptoms” was bigger (n=200), more rigorous, and used better bacterial sequencing techniques than past studies.
A lactobacillus gut bacteria (Dr.-Horst-Neve-Max-Rubner-Institut-CC-BY-SA-3.gif). This study found a reduced diversity of gut bacteria in ME/CFS.
Ian Lipkin’s team involved seemingly everyone possible (Mady Hornig, Anthony Komaroff, Susan Levine, Lucinda Bateman, Suzanne Vernon, Nancy Klimas, Jose Montoya, Dan Peterson) in this big NIH-funded study. The study was not just big – it was long as well. Study participants provided stool samples 4x’s over a year and completed a multidimensional fatigue inventory each time to boot.
Plus, following past studies from this group – the study assessed the effect irritable bowel syndrome – a common but certainly not universal comorbid condition found in ME/CFS – has on gut flora. Finally, as it did in a past study, this new study added metabolomics to the mix as well.
The study also used a technique that appears to be new to ME/CFS gut studies called “
shotgun metagenomics sequencing” which allows researchers to comprehensively sample all the genes in all the organisms found in a complex environment. This technique allows researchers to detect bacterial species that other methods are likely to miss.
This study provides us clearly our best look yet at the gut bacteria in chronic fatigue syndrome (ME/CFS).
The Irritable Bowel Syndrome (IBS) Connection
“Both our current and prior fecal shotgun metagenomic study support the notion that IBS morbidity must be carefully considered in future ME/CFS microbiome studies”. The authors
Irritable bowel syndrome (IBS) is characterized by symptoms like abdominal pain, cramping or bloating usually associated with a bowel movement, changes in the appearance of bowel movements (ranging from hard-packed feces to diarrhea), and changes in how often you’re having a bowel movement. About a third of the ME/CFS patients in the study had IBS.
A Less Diverse Ecosystem
Having IBS made a real difference. Specifically, the differences in the alpha diversity of the gut bacteria largely reflected whether one had IBS or not.
Alpha diversity is a term borrowed from ecology that measures the richness or diversity of complex ecosystems (like a forest or a gut). Like a forest, the higher the species richness or alpha diversity of the bacteria in the gut the more resilient, healthy, and productive the gut should be
The bacteria in our guts form an ecosystem. This study suggests that some ME/CFS patients’ gut ecosystems are less diverse and less resilient (“Wild Garden of the gut bacteria” – from Wikimedia Commons”)
Alpha diversity isn’t the only measure of gut health but it is an important one. One Ph.D. student reported that “
High gut alpha diversity has been linked to a healthy state in so many studies that it has become common knowledge in microbiome circles.”
Having IBS had a dramatic influence on alpha diversity with only the ME/CFS +IBS having a reduced alpha diversity compared to healthy controls.
Beta diversity can also be used to assess gut health. The beta diversity test compared the difference between the microbiome species found in the presumably healthier guts of the healthy controls with the ME/CFS patients with and without IBS. In contrast to the alpha diversity finding, both ME/CFS cohorts (those with and without IBS), had altered beta diversity; i.e. whether they had IBS or not, the flora in the ME/CFS patients’ guts was markedly different than the flora found in the healthy controls’ guts.
“A Potential Biosensor of Human Health” Reduced
The study found that the relative abundance of two species (
E. rectale and
Faecalibacterium prausnitzii ) and six genera (
Eubacterium,
Faecalibacterium, Dorea,
Roseburia,
Gemmiger ) differed in relative abundance in ME/CFS compared to healthy control subjects.
F. prausnitizi stood out because it’s been found reduced in at least one fibromyalgia and two ME/CFS studies. This odd bacterium, which does not produce spores, and moves around very little, makes up a full 5% of the bacteria found in our guts. Through its fermentation of dietary fiber,
F. prausnitizi produces
butyrate and other
short-chain fatty acids, and an important anti-inflammatory product. It’s been called “a potential biosensor of human health”.
The authors noted that low fecal
F. prausnitzii levels have been found in inflammatory bowel disease (IBD), IBS, celiac disease, colorectal cancer, obesity, in people with COVID, and in long COVID. The Flemish gut project found that
Faecalibacterium and
Coprococcus bacteria were associated with higher quality of life scores, and reduced levels of
F prausnitizii have been associated with fatigue in inflammatory bowel disease.
Reduced Butyrate Levels – A Seminal Gut Finding in ME/CFS
Fatigue was certainly the byword in this study as lower butyrate levels were associated with more fatigue and both general and/or physical fatigue were particularly associated with reduced levels of
F. prausnitz,
Coprococcus, and a few other bacteria.
Butyrate’s becoming a pretty big deal in ME/CFS gut studies. It’s the primary energy source for the endothelial cells lining the gut – an interesting finding given all the focus on the endothelial cells lining the blood vessels in ME/CFS and long COVID. Given the strong possibility that leaky gut is present in ME/CFS, it’s intriguing that butyrate protects the gut lining. Its support of regulatory T-cells and its anti-inflammatory activity helps keep the immune system in check as well.
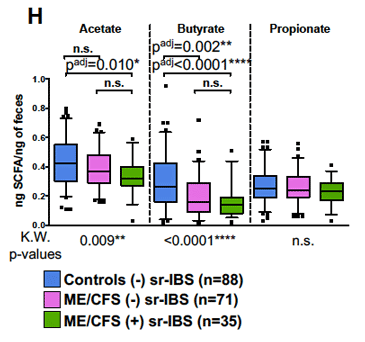
Dramatically reduced butyrate levels (middle column) in ME/CFS patients (with IBS – green; without IBS – violet) compared to healthy controls.
Reduced butyrate levels in ME/CFS then, could result in an inflammatory state in the gut and a weakened gut barrier to boot; which is perhaps just the right combination to spark an inflammatory response that ranges from the gut all the way to the brain.
The deeper the researchers dug, the more solid the low butyrate finding became. A metagenomic analysis snagged one source of the butyrate problem when it found that people with ME/CFS tended to be deficient in one of the two genes (the “but” gene) used to produce butyrate using the acetyl-CoA pathway gene. A functional analysis then indicated that the bacteria using that gene were the same bacteria that people with ME/CFS were missing.
The butyrate connection got even stronger when the authors found reduced levels of a substance called acetate. Because butyrate-producing bacteria need acetate to produce butyrate, the acetate deficiency fit the bill.
The Gist
- Ian Lipkin’s group presented the findings of the largest gut study ever done in ME/CFS.
- The study found that having ME/CFS and irritable bowel syndrome – reduced the “alpha diversity” or richness of their gut flora – resulting presumably in less productivity and resilience. Just having ME/CFS, on the other hand, did not.
- As several other studies have found the bacteria that produce butyrate – an anti-inflammatory that provides essential energy resources to the endothelial cells linking the gut wall – were deficient in ME/CFS. Low levels of butyrate were associated with greater fatigue as well.
- With this study, three different methodological approaches have identified low butyrate levels in ME/CFS patients’ guts – making it one of the more solid findings in all of ME/CFS.
- Low levels of butyrate could result in inflammation, weakened gut linings, and leaky gut – potentially producing systemic inflammation that reaches all the way to the central nervous system.
- This also makes this study the third to find reductions of a specific butyrate producer called F. prausnitizi. Low levels of F. prausnitizi , which has been called a potential biosensor of health, have been found in a number of chronic diseases including IBS, IBD, and fibromyalgia.
- Because acetate is needed to produce butyrate, the reduced acetate levels found made sense as well.
- What was causing the higher-than-normal levels of bacteria in ME/CFS patients’ feces was unclear.
- Recent studies which have shown that reduced activity levels are associated with reduced levels of butyrate bacteria suggest one reason why butyrate-producing bacteria may be low in ME/CFS.
- People with ME/CFS could be caught in a kind of low butyrate “loop” where low butyrate levels spark inflammation, which produces fatigue thus reducing activity levels, reducing butyrate levels further, etc.
- GIven that, it’s possible that increasing gut butyrate levels in ME/CFS could reduce inflammation, reduce fatigue, increase activity levels, etc.
- An upcoming blog will focus on increasing butyrate levels.
Don't Miss Another Blog!
Make sure you don’t miss another one by registering for our free
ME/CFS and Fibromyalgia blogs here...
Digging even further into the butyrate mystery, the authors found that one of the four butyrate-producing pathways – the dominant one called the CoA pathway – was responsible for the butyrate deficiency found.
The fact that three different techniques (qPCR, metabolomics, metagenomics) have now found low butyrate-producing bacteria in ME/CFS makes the butyrate gut finding possibly one of the most solid findings in ME/CFS.
The fact that more people with ME/CFS than healthy controls (10%-2%) were taking supplements (prebiotic fiber) which should have
enhanced their levels of butyrate-producing bacteria present only increased the mystery about the low butyrate levels studies have consistently found in ME/CFS.
Bacteria Packed Feces
Another interesting finding concerned the fact that the feces of the ME/CFS patients were packed with more bacteria than normal. The authors discarded antibiotic use, acute malnutrition, and inflammatory bowel diseases as possible causes and suggested several possibilities: low levels of FODMAPs (fermentable oligo-, di-,539 mono-saccharides, and polyols) foods, small intestinal bacterial overgrowth (SIBO), greater bacterial “washout” or the loss of bacteria that adhere to the mucosal walls.
FODMAPS diets can be helpful in IBS and SIBO appears to be common in ME/CFS. (The idea of malnutrition was intriguing – at least to this layman’s mind – given that the metabolomic findings in ME/CFS mirror those found in starvation and the possibility that the cells of ME/CFS patients may be starving in the midst of plenty. Could people with ME/CFS also not be utilizing all the nutrients in their food?)
The Activity Question
We don’t know why the butyrate-producing bacteria are so low in ME/CFS but low levels of physical activity may come into play.
Recent studies (mostly done on rodents) indicate that physical exercise stimulates the same butyrate-producing bacteria that are deficient in ME/CFS.
It’s not clear why this is so but numerous possibilities (reduced gut motility, reduced blood flows to the gut, circulation of bile acids, etc.) exist. The association this study found between reduced butyrate levels and fatigue could, then, be caused by the lower activity levels that more fatigued people with ME/CFS engage in. Clearly, we need gut studies that also assess activity levels to address this issue.
It’s clear to me personally, though, that low activity levels are not responsible for all the gut perturbations in ME/CFS as I am not deconditioned (and have the Oura stats to prove it), yet I still intermittently suffer from irritable bowel syndrome.
In a sense, the activity question doesn’t matter. Given that the guts of people with ME/CFS and perhaps fibromyalgia and long-COVID patients have low butyrate levels which could result in gut inflammation, leaky gut problems, and perhaps even systemic and neuroinflammation – the question is what to do about them?
It’s possible that people with these diseases are caught in a kind of low butyrate loop: their lowered activity levels (or something else) resulted in reduced levels of butyrate-producing bacteria – which then produced inflammation and leaky gut (particularly as a result of exercise), which resulted in more fatigue and less exercise, which further reduced their butyrate levels, and on and on.
One way out of this loop might be to increase butyrate levels, thus reducing the inflammation and leaky gut (particularly after exercise) allowing people with ME/CFS to be more active, resulting in more butyrate production, higher activity levels, and on and on.
- Coming up – increasing acetate and butyrate levels in the gut for better health


 Willem M De Vos
Willem M De Vos Huibert Michiel Vriesendorp
Huibert Michiel Vriesendorp Peter Heidt
Peter Heidt Mahesh Anantha Narayanan
Mahesh Anantha Narayanan Toufik Mahfood Haddad
Toufik Mahfood Haddad Jon Iredell
Jon Iredell Bruce E Hirsch
Bruce E Hirsch Gerard Honig
Gerard Honig