Murph
:)
- Messages
- 1,805
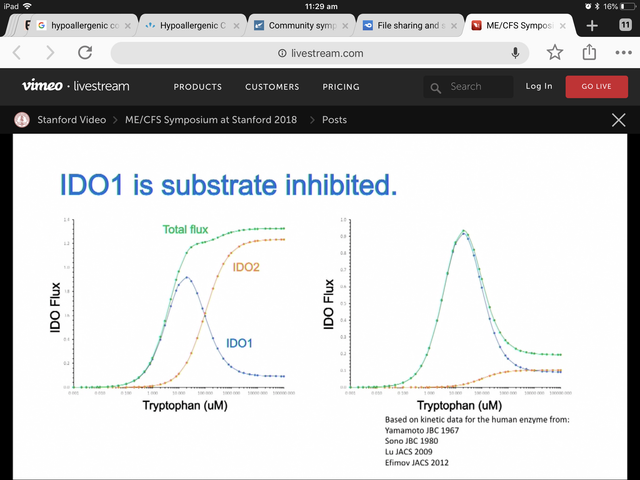
The possible problem, though, is that the Michaelis-Menten chart that Phair presented appears to indicate they may be giving IDO2 a much greater affinity (~200 μM) for tryptophan than what the literature is saying (i.e., ~7000 to 9000 μM).
Like you, I could find little in the literature that suggested humans ever actually use IDO2. (More precisely, I found nothing, but I didn't read all the literature!) Now, it is highly evolutionarily conserved, but perhaps there is another reason for that.
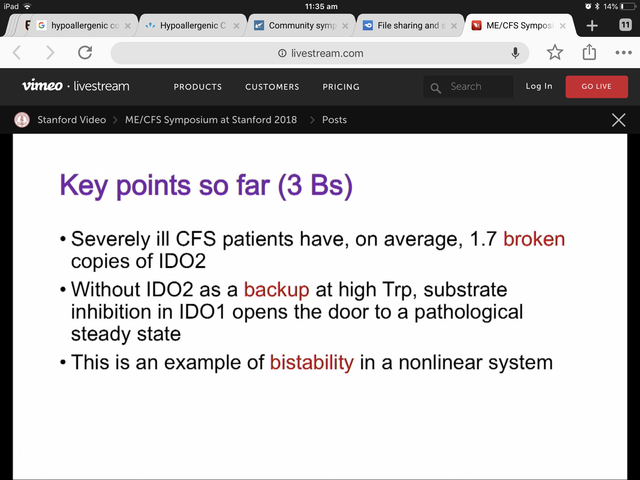
So the mystery of whether IDO2 mutations matter persists.
I am not yet convinced by the SNP frequency in the severely ill study. 55% (sample) vs 42% (population) frequency in a common mutation with a sample of 20? While other genes affecting the same enzyme show a lower frequency than the population?
Bonferroni: LOL.
Bayes:
(being snarky here but I do acknowledge that Phair is no slouch!)
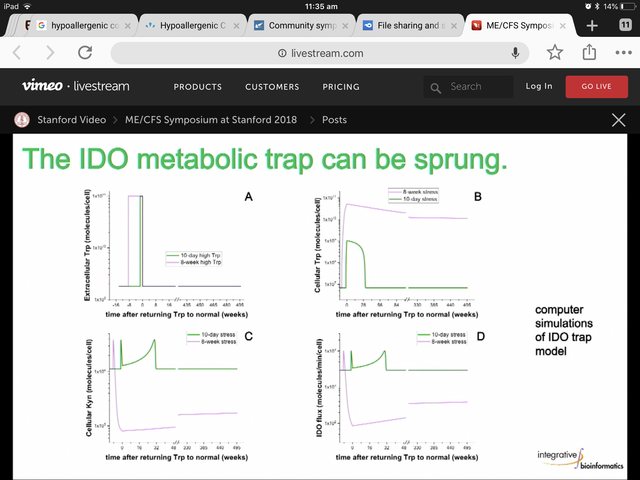
Anyway, in my experience he loves an email about his theories, and I expect he has got many far stupider questions than yours, @nandixon. (Although he may be suffering more inbox pressure now than pre-symposium!) Why not drop him a line and let us know if he can explain why that flux graph looks as it does? You can find his email in this post by Cort. (I've been told bots roam this site harvesting emails so I'm not posting it here.)
Last edited: