You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
T3 intracellular calcium and caffeine
- Thread starter pattismith
- Start date
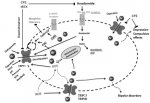
Putting G protein–coupled receptor-mediated activation of phospholipase C in the limelight
For example, receptors coupled to PLC activation could be the heptahelical, G protein–coupled kind, such as the angiotensin II, the V2 vasopressin, or the m1, m3 muscarinic, and the α1-adrenergic receptors
For a relatively long period of time, PLC activation was thought to serve the sole purpose of generating two messenger molecules, inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol by hydrolyzing the small pool of PtdIns(4,5)P2 in the plasma membrane. Ins(1,4,5)P3 liberates Ca2+ from internal Ca2+ stores and hence indirectly controls Ca2+ entry via store-operated Ca2+ entry pathway(s), and diacylglycerol stimulates the activity of protein kinase C enzymes, thereby initiating a cascade of downstream signaling responses
For example, receptors coupled to PLC activation could be the heptahelical, G protein–coupled kind, such as the angiotensin II, the V2 vasopressin, or the m1, m3 muscarinic, and the α1-adrenergic receptors
For a relatively long period of time, PLC activation was thought to serve the sole purpose of generating two messenger molecules, inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol by hydrolyzing the small pool of PtdIns(4,5)P2 in the plasma membrane. Ins(1,4,5)P3 liberates Ca2+ from internal Ca2+ stores and hence indirectly controls Ca2+ entry via store-operated Ca2+ entry pathway(s), and diacylglycerol stimulates the activity of protein kinase C enzymes, thereby initiating a cascade of downstream signaling responses
What if aside from cfs-antibodies blocking the activation of PL-C, there was some aberration in the IP3-receptive ER/SR calcium channels that slowed the influx of Ca2+ to the cytosol, thereby cutting the PL-C Cascade.
When PL-C is activated IP3 and DAG are formed. DAG and Ca2+ together serve to activate protein kinase C.
An ineffective release of Ca2+ following PL-C would not only cut the positive feedback short, it would doubly hinder the activation of PKC
When PL-C is activated IP3 and DAG are formed. DAG and Ca2+ together serve to activate protein kinase C.
An ineffective release of Ca2+ following PL-C would not only cut the positive feedback short, it would doubly hinder the activation of PKC
Last edited:
Iritu1021
Breaking Through The Fog
- Messages
- 586
So M1 antibodies inhibit the receptor, and M1 activation can activate the Phospholipase C pathway.
Also, the activation of PL-C is potentiated by the immediate feedback of Ca2+ rising. If the rise fails or slows, this will hamper PL-C activation .
Where do you see that they are inhibitory antibodies? I looked through the article and it sounded like the researchers are not yet sure whether they are functional or in which way they function.
I know that hypothyroidism was a trigger for my CFS but I also know that pushing intracellular release without addressing the membrane influx sooner or later leads to trading one set of issues for another. The amount of time before it takes for things to head south seems to be directly proportional to my ER/SR reserves.
I also thought this was interesting from the article - which regulatory functions do they mean?
What are potential mechanisms leading to induction of β AdR and M AChR antibodies in CFS patients? There is evidence that a subset of patients experienced major distressing life events before CFS onset and that CFS is frequently triggered by an infection. Thus it is tempting to speculate that chronic adrenergic stimulation may lead to conformational changes of receptors resulting in more immunogenic epitopes and that infection-triggered immune activation induces the autoantibody response. It is also conceivable that low level non-pathogenic β AdR and M AChR antibodies exerting regulatory functions are already present in healthy subjects.
Gingergrrl
Senior Member
- Messages
- 16,171
Did the level of your calcium blocking antibody go down after rituximab?
We don't actually know yet but I just did the Mayo DYS1 Panel yesterday which includes the calcium channel autoantibodies (P/Q and N-type) and my doctor will have the results in about 3 wks. I learned I had this autoantibody (and several others) in 2016 pre-treatment. Now after 2 yrs of IVIG and one year of Ritux, we are re-testing the autoantibodies, starting with the panel I mentioned to compare.
Your question sure makes sense but I don't know what to make of it either. Did you say that you had different types of issues before and after IVIG?
Even though I had some symptom improvement from various meds and treatments, I will extremely ill when I began IVIG. It put my MCAS and allergic reactions to food, smells, chemicals, etc, 100% into remission and they never returned. It also completely eliminated an insanely exaggerated startle response that I had which we believed linked to the anti GAD65 autoantibody. It also started to improve my muscle strength which was extremely weak (but not even close to the point that I could walk without wheelchair).
Which symptoms were helped by IVIG and which ones by rituximab - were they the same or different?
There is no way to ever know with 100% certainty b/c the two treatments overlapped (except for now, I am done with IVIG and only doing Ritux). But after we added Ritux, over the course of the next year, my muscle strength continued to improve, my breathing improved, my POTS improved, and when I got to the 5th Rituximab infusion (of a series of six), I no longer needed to use wheelchair that I had used 24/7 for 3.5 years. I was able to breathe, stand, and walk normally. I also was able to walk my dog again, drive my car again, and live a normal life. The most intractable symptom (the POTS reactions which I still triggered by raising my arms or bending to the floor, etc) also completely went away. I probably could still trigger one if I really tried but I don't do anything too crazy
I will consider this remission permanent if it continues once I have stopped Rituximab. But each infusion can last for 3 months (in responders) so this current one will be effective until early Nov and then I will have at least one maintenance infusion (and most likely more).
What are potential mechanisms leading to induction of β AdR and M AChR antibodies in CFS patients?
I also test positive for the B AdR and M AChR autoantibodies but didn't understand the above posts... do these autoantibodies also link to calcium in some way?
Here's a review about IP3Rs and the proteins with which they interact:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4887697/
There's certainly something like this for ryanodine receptors.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4887697/
There's certainly something like this for ryanodine receptors.
Where do you see that they are inhibitory antibodies? I looked through the article and it sounded like the researchers are not yet sure whether they are functional or in which way they function.
I know that hypothyroidism was a trigger for my CFS but I also know that pushing intracellular release without addressing the membrane influx sooner or later leads to trading one set of issues for another. The amount of time before it takes for things to head south seems to be directly proportional to my ER/SR reserves.
I also thought this was interesting from the article - which regulatory functions do they mean?
What are potential mechanisms leading to induction of β AdR and M AChR antibodies in CFS patients? There is evidence that a subset of patients experienced major distressing life events before CFS onset and that CFS is frequently triggered by an infection. Thus it is tempting to speculate that chronic adrenergic stimulation may lead to conformational changes of receptors resulting in more immunogenic epitopes and that infection-triggered immune activation induces the autoantibody response. It is also conceivable that low level non-pathogenic β AdR and M AChR antibodies exerting regulatory functions are already present in healthy subjects.
To be honest, I kinda assumed the antibodies would have an antagonistic behaviour. On further examination, this doesnt seem to be true:
Antibodies against cerebral M1 cholinergic muscarinic receptor from schizophrenic patients: molecular interaction.
the corresponding affinity-purified antipeptide Ab displayed an agonistic-like activity associated to specific receptor activation
Iritu1021
Breaking Through The Fog
- Messages
- 586
In this case, it could be plausible that these elevated antibodies might actually be a compensatory attempt to regulate calcium influx by activating M1 receptor.To be honest, I kinda assumed the antibodies would have an antagonistic behaviour. On further examination, this doesnt seem to be true:
Antibodies against cerebral M1 cholinergic muscarinic receptor from schizophrenic patients: molecular interaction.
the corresponding affinity-purified antipeptide Ab displayed an agonistic-like activity associated to specific receptor activation
It's so hard to tell what's the chicken and what's the egg.
"The amount of time before it takes for things to head south seems to be directly proportional to my ER/SR reserves"
This means not only that there is an insufficient release of Ca into the cytosol, but also that ER/SR stores don't replenish in a normal fashion.
Maybe the ER/SR doesnt pump out more calcium because it wont be able to replenish. Do you know what regulates the amount of Ca stored in ER/SR?
This means not only that there is an insufficient release of Ca into the cytosol, but also that ER/SR stores don't replenish in a normal fashion.
Maybe the ER/SR doesnt pump out more calcium because it wont be able to replenish. Do you know what regulates the amount of Ca stored in ER/SR?
Iritu1021
Breaking Through The Fog
- Messages
- 586
I was thinking about that too - I don't know. Going back to my summary, I think my defect is most likely to be at the A or most likely at B level, so going directly at C without addressing the issue with Ca2+ supply is bound to result in eventual depletion."The amount of time before it takes for things to head south seems to be directly proportional to my ER/SR reserves"
This means not only that there is an insufficient release of Ca into the cytosol, but also that ER/SR stores don't replenish in a normal fashion.
Maybe the ER/SR doesnt pump out more calcium because it wont be able to replenish. Do you know what regulates the amount of Ca stored in ER/SR?
The ryanodine and ip3 receptors (types 1-3), along with inositol 1,4,5 trisphosphate (and Ca2+), which de-/activate the calcium channel, but also other proteins can activate the receptors (see the review paper for IP3R I linked above).Do you know what regulates the amount of Ca stored in ER/SR?
(In my case, the type 3 Ip3R seems to be dysfunctional due to a pathogenic mutation in ITPR3. It has to be found what the consequences are. Of course, especially after what I have read here and elsewhere, I think it plausible that this might be the "underlying problem". Maybe others have comparable problems that lead to comparable symptoms?)
Last edited:
Iritu1021
Breaking Through The Fog
- Messages
- 586
@Inara, how did you arrive at that conclusion - through genetic testing? I think I might have problem with IP3 too.The ryanodine and ip3 receptors (types 1-3), along with inositol 1,4,5 trisphosphate (and Ca2+), which de-/activate the calcium channel, but also other proteins can activate the receptors (see the review paper for IP3R I linked above).
(In my case, the type 3 Ip3R seems to be dysfunctional due to a pathogenic mutation in ITPR3. It has to be found what the consequences are. Of course, especially after what I have read here and elsewhere, I think it plausible that this might be the "underlying problem". Maybe others have comparable problems that lead to comparable symptoms?)
I'm reading your paper:
Although IP3 initiates Ca2+ signals by stimulating Ca2+ release from intracellular stores, the signals are sustained by Ca2+ entry across the plasma membrane. That too is indirectly regulated by IP3, because store‐operated Ca2+ entry is stimulated by loss of Ca2+ from the ER (Parekh & Putney, 2005; Lewis, 2012).
Maybe that's my issue - that I don't have a sustained signal.
As for ryanodine receptors I found this and it made me wonder if this is the basis for infamous coffee enemas that are so popular in alternative medicine (I never tried):
RyRs are activated by millimolar caffeine concentrations. High (greater than 5 mmol/L) caffeine concentrations cause a pronounced increase (from micromolar to picomolar) in the sensitivity of RyRs to Ca2+ in the presence of caffeine, such that basal Ca2+ concentrations become activatory. At low millimolar caffeine concentrations, the receptor opens in a quantal way, but has complicated behavior in terms of repeated use of caffeine or dependence on cytosolic or luminal calcium concentrations.
pattismith
Senior Member
- Messages
- 3,990
@Inara @Iritu1021
did you see that two months after I discovered this positive effect I had with T3 + caffeine, there was this paper published about deplete intracellular cytoplasmic calcium in CFS and a link with the TRPM3:
https://forums.phoenixrising.me/ind...ural-killer-cells-from-cfs-me-patients.61238/
This TRP channel could be involved in the initial problem as well
A preliminary paper was published in december 2017, and was discussed here (so that I can't claim to have discovered first the cytoplasmic calcium depletion )
)
did you see that two months after I discovered this positive effect I had with T3 + caffeine, there was this paper published about deplete intracellular cytoplasmic calcium in CFS and a link with the TRPM3:
https://forums.phoenixrising.me/ind...ural-killer-cells-from-cfs-me-patients.61238/
This TRP channel could be involved in the initial problem as well
A preliminary paper was published in december 2017, and was discussed here (so that I can't claim to have discovered first the cytoplasmic calcium depletion
Last edited:
Iritu1021
Breaking Through The Fog
- Messages
- 586
@Inara @Iritu1021
did you see that two months after I discovered this positive effect I had with T3 + caffeine, there was this paper published about deplete intracellular cytoplasmic calcium in CFS and a link with the TRPM3:
https://forums.phoenixrising.me/ind...ural-killer-cells-from-cfs-me-patients.61238/
This TRP channel could be involved in the initial problem as well
A preliminary paper was published in december 2017, and was discussed here (so that I can't claim to have discovered first the cytoplasmic calcium depletion)
I've heard about that paper but I'm able to understand the significance of it much better now that we've had this discussion.
There's a lot of discussion about the role of calcium chanels in psychiatric community as well.
Here's a summary article https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4598437/
Here are some highlights:
Thus, TRPM2 is a highly relevant pathophysiological candidate for bipolar disorders, as its dysfunction may affect calcium ion signaling.
In addition, chronic lithium treatment of the cell lines in patients with bipolar disorder significantly decreased TRPC3 protein levels but not TRPC1 protein and mRNA levels, as determined with immunoblotting [84]. These results suggest that lithium may correct abnormal calcium ion homeostasis through down-regulation of TRPC3 channels in the cell lines in bipolar disorders [84]
(I don't fully understand what that means about lithium)
Iritu1021
Breaking Through The Fog
- Messages
- 586
here's a good diagram from that article.
@debored13 , this might explain why gabapentin worked for you through calcium channel mechanism
@debored13 , this might explain why gabapentin worked for you through calcium channel mechanism
Attachments
A genetist made an exome analysis and this mutation was marked as relevant. It turned out it's inherited and the genetist said it's significant. Now other family members get tested and we're trying to identify the phenotype (together with other doctors/hopefully researchers).@Inara, how did you arrive at that conclusion - through genetic testing? I think I might have problem with IP3 too.
I also did a commercially whole genome sequencing.
Regarding caffeine and ryanidine receptors, it's possible that @pattismith's observation is due to that.
Very good thought!Maybe that's my issue - that I don't have a sustained signal.
@pattismith, the TRPM channels are something else. But I could imagine if you have "minor mutations" there, too, that the problem might get enhanced. I will take a closer look at that - wasn't there an interaction between IPR3 and TPRM, too? I think I mix something.
I know I have (genetic) magnesium deficiency, and the WGS results suggest I have known mutations that are correlated with magnesium/calcium deficiency (renal loss).
Gingergrrl
Senior Member
- Messages
- 16,171
I can’t add anything of use but am still following this thread and it’s very interesting b/c I am convinced calcium plays a major role in our illnesses (even if it’s in several different ways for different people or sub-groups).
Interesting also re: Gabapentin and it is part of a whole group of meds I was told not to take b/c they block the calcium channel. (I don’t take that one anyway but sometimes it is hard to figure out if a med is in that category)!
Interesting also re: Gabapentin and it is part of a whole group of meds I was told not to take b/c they block the calcium channel. (I don’t take that one anyway but sometimes it is hard to figure out if a med is in that category)!
Iritu1021
Breaking Through The Fog
- Messages
- 586
A genetist made an exome analysis and this mutation was marked as relevant. It turned out it's inherited and the genetist said it's significant. Now other family members get tested and we're trying to identify the phenotype (together with other doctors/hopefully researchers)
I also had my whole genome sequenced a while back but my geneticist only looked for Ehlers-Danlos genes at the time so he probably missed my calcium aberrations or didn't know what to look for. If you find any researchers who study calcium genetics, I'd like to get in on that!
Iritu1021
Breaking Through The Fog
- Messages
- 586
J Neurosci. 2009 Feb 18;29(7):2053-63. doi: 10.1523/JNEUROSCI.4212-08.2009.
Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels.
Ryan D1, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B.
Author information
Abstract
Cannabinoids and the endocannabinoid system have attracted considerable interest for therapeutic applications. Nevertheless, the mechanism of action of one of the main nonpsychoactive phytocannabinoids, cannabidiol (CBD), remains elusive despite potentially beneficial properties as an anti-convulsant and neuroprotectant. Here, we characterize the mechanisms by which CBD regulates Ca(2+) homeostasis and mediates neuroprotection in neuronal preparations. Imaging studies in hippocampal cultures using fura-2 AM suggested that CBD-mediated Ca(2+) regulation is bidirectional, depending on the excitability of cells. Under physiological K(+)/Ca(2+) levels, CBD caused a subtle rise in [Ca(2+)](i), whereas CBD reduced [Ca(2+)](i) and prevented Ca(2+) oscillations under high-excitability conditions (high K(+) or exposure to the K(+) channel antagonist 4AP). Regulation of [Ca(2+)](i) was not primarily mediated by interactions with ryanodine or IP(3) receptors of the endoplasmic reticulum. Instead, dual-calcium imaging experiments with a cytosolic (fura-2 AM) and a mitochondrial (Rhod-FF, AM) fluorophore implied that mitochondria act as sinks and sources for CBD's [Ca(2+)](i) regulation. Application of carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and the mitochondrial Na(+)/Ca(2+) exchange inhibitor, CGP 37157, but not the mitochondrial permeability transition pore inhibitor cyclosporin A, prevented subsequent CBD-induced Ca(2+) responses. In established human neuroblastoma cell lines (SH-SY5Y) treated with mitochondrial toxins, CBD (0.1 and 1 microm) was neuroprotective against the uncoupler FCCP (53% protection), and modestly protective against hydrogen peroxide- (16%) and oligomycin- (15%) mediated cell death, a pattern also confirmed in cultured hippocampal neurons. Thus, under pathological conditions involving mitochondrial dysfunction and Ca(2+) dysregulation, CBD may prove beneficial in preventing apoptotic signaling via a restoration of Ca(2+) homeostasis.