Ponderings and speculations about purinergic signaling, in pursuit of a unified ME/CFS theory, Part 1
Introduction
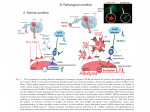
ATP, while inside the cell serving for energy transport, when outside of the cells has signaling functions. Those functions differ between cell types, but a lot of it is pro-inflammatory, where extracellular ATP serves as a danger signal, so-called Damage Associated Molecular Pattern, DAMP. This is because ATP should normally be mostly contained inside the cells, and spills outside when a cell is ruptured, either by mechanical means or by an invading pathogen, therefore making a good danger signal to start the immune response we know as inflammation.
Dr. Naviaux proposed that overactive purinergic signaling sustains many chronic diseases, including ME/CFS and autism. In the case of autism he's also proven that this seems to be the case, by causing symptom remissions with IV administration the anti-purinergic drug suramin. He thinks the same might be happening in ME/CFS, and Alan Light's research which indicates that extracellular ATP is a key ingredient in facilitating the feeling of fatigue itself, supports this hypothesis.
For me, Naviaux's theories seemed fascinating, and made a lot of sense, but many questions arose, like how do we get different diseases from a unified danger response, how does it tie in with other observations in ME/CFS, how exactly does this cause our symptoms, etcetera. Naviaux never explained his theories in detail, only gave an incredibly simplified version at the Stanford symposium. Some details can be understood reading his papers about CDR and oxidative shielding, but those prompted even more questions in me. So I did the crazy thing and decided to dive into Google Scholar trying to understand this stuff myself by reading related papers. As it currently stands, I am very far from any form of full understanding of this subject, but nevertheless, I wanted to share my findings with you all, first to save some trouble other people interested in it, second because there are people here who understand biochemistry better than me (Im gonna tag a few -
@nandixon,
@Hip,
@JaimeS), and might help me in refining this. And third, because our amazing Ron Davis apparently likes to get ideas from the community, which is why I'm tagging
@Janet Dafoe (Rose49) to pass this post to him. I imagine he heard a lot of this from Naviaux and other scientists, but maybe a thing or two will spark a novel thought. I'm sorry it's so long, but I assure you I put a lot of thought in which of my findings include here and which not.
Biochemical background for the uninitiated
Purinergic receptors, which detect the presence of ATP and other purines outside the cell, consist of P1, P2X and P2Y families. P2X receptors are the ones most specific to ATP and seem to be most well studied.
ATP release from the cells can also occur without the cell rupturing, but rather to send the danger signal on purpose. ATP can be released vesicularly, but the more prominent and well studied method seem to be pannexin and connexin hemichannels, particularly Panx1. It's basically a protein on the surface of the cell, which when activated creates a large pore, permeable to molecules up to 900Da in size, including ATP. Those have been shown to open in various cell types in response to stressful stimuli - mechanical stretching
[source], hypoxia
[source]. This is in line with the model of eATP serving as a danger signal.
Interestingly, in some cells, Panx1 channels can be directly opened by activation of the P2X7 receptor.
[source] They kinda bundle together and activation of the P2X7 activates Panx1 as well. By this mechanism, when a cell senses large amounts of extracellular ATP, it also releases ATP to propagate the danger signal to the next set of neighboring cells.
So what does eATP do to the cells, other than make them release more ATP? Well, depends on the cell. In this Part 1, I will mostly focus on the immune system.
When a pathogen enters the body, macrophages and dendritic cells (which are in the tissues) detect them through Toll-Like Receptors, becoming activated. They then secrete Il-1β and TNF-α, major cytokines that start the inflammatory response. This secretion process is hugely dependent on co-stimulation by eATP mainly through the P2X7 receptor. eATP also serves as a signal for those cells to migrate to the infection site in the first place.
[source] It also is a necessary factor for neutrophils to get there, as it potentiates the Il-8 mediated chemotaxis through the P2Y2 receptors.
[source] The secretion of Il-8 from activated monocytes also requires P2Y2 and P2Y6 stimulation in them.
[source]
Macrophages and dendritic cells engulf the pathogens, break them down, and present their peptides on MHC proteins to T cells, activating them if their receptors correspond to the presented peptide. T cells express P2X1, P2X4, P2X7 receptors, and Panx1. All of those, with the exception of P2X7, translocate to the immune synapse (the connection between the T cell and the antigen presenting cell), and ATP is released from the T cell, amplifying the activation signal by acting on its own P2 receptors.
[source] The activated T cell produces IL-2, which acts on the T cell itself, triggering it's clonal expansion. Presence of eATP outside of the immune synapse, acting on P2X7, can also increase IL-2 secretion in activated T cells.
[source] Th cells then activate B cells which produce antibodies, and cytotoxic T cells go killing infected cells in the body. B cells also express purinoreceptors, but the effects of their activation have not been studied.
Basically, extracellular ATP is an important co-factor in almost every step of immune activation, having pro-inflammatory effects. Except for NK cells. For NK cells, the presence of extracellular ATP actually reduces their cytotoxic activity.
[source] (Sound familiar?)
The good stuff
ATP is not the only pro-inflammatory molecule, the immune system has a large number of cytokines to regulate itself as well as other Damage Associated Molecular Patterns, and Pathogen Associated Molecular Patterns. In a lot of studies assessing purinergic signaling in immune cells, they found that many immune cell types have two or more pathways for activation of their primary functions - one through purinergic receptor stimulation by ATP, and other through direct contact with a pathogen, or through cytokines. Those pathways are often needed to act in synergy to achieve a full-blown immune activation.
[source] Given all this, it is possible, that a "kind of" inflammatory response is perpetuated in ME/CFS by purinergic signaling. There was an infection (or other triggers), which initiated classical immune response, which resolved the infection, and initiated anti-inflammatory immune resolution, but that resolution succeeded only partially. We might not see very elevated cytokines in ME/CFS because that kind of signaling has already been mostly resolved, while eATP pro-inflammatory signaling still persists. Now, obviously those are not fully separate, but it's very possible they're not always completely in sync either. It could be that they're only kinda-connected. This could explain the finding of Montoya's and Mark Davis's cytokine profiling, that more severe patients have higher pro-inflammatory cytokines, but are still not high enough to distinguish from controls.
[source] Those severe patients would have stronger eATP-mediated inflammation, which elicits higher cytokine levels, but because that connection is weak, the cytokine levels don't quite raise above the norm.
But why doesn't this happen in healthy people? What's the difference between them and ME/CFS patients? To answer this, there is a simple approach and a complex approach.
The simple approach is to go "Wait, eATP makes cells release more ATP? This is a positive feedback loop. There must be some inhibitory mechanism of this, otherwise the whole immune system would go into runaway endless inflammation". And there is. ATP can be hydrolyzed to AMP and then to adenosine, by enzymes called nucleotidases. Nucleotidases present outside the cell, or ecto-nucleotidases, are indeed expressed by many cells which also have purinergic receptors. The main such ecto-nucleotidase, which seem to be responsible for most of eATP breakdown is NTPDase1, also called CD39.
[source1][source2] It was shown in many studies to downregulate purinergic signaling, first by reducing eATP concentrations, and second, along with 5'-NT (CD73), it produces adenosine, activating some of the P1 receptors, which have anti-inflammatory functions in many immune cells.
[source]
The obvious hypothesis from this is that CD39 function is impaired in ME/CFS. Now, I'm unsure of this because I don't know the specifics of the test, but I'm under the impression that this, to some extent, can be tested in Ron's impedance meter. First, you can introduce recombinant CD39 into the plasma and see if it reduces the signal in ME/CFS cells. Second, you can take healthy cells (in healthy plasma) and treat them with the CD39 inhibitor, ARL 67156, and see if the impedance response starts resembling that of ME/CFS. It might be necessary to add extracellular ATP also, to mimic nearby cells secreting it, as it (presumably) happens in ME/CFS. You'd do two variants - one with just eATP, and one with eATP and ARL 67156. Another way might be to add a non-hydrolyzable ATP derivative, which would activate P2 receptors, but won't be hydrolyzed by CD39. The obvious limitation of this is that it only tests white blood cells, and what's happening in the tissues might be a different story.
So, if CD39 activity really was inhibited in ME/CFS, what could be the reason? I see three possibilities, and to talk about them, I first need to talk about Treg cells. T regulatory cells, formerly known as T suppressive cells, are responsible for control and suppression of the immune response. They are also the most confusing little shits ever.
[source] To a lot of questions about them, the answer is "data is not conclusive". But we do know that they are the main source of CD39 and CD73 in the immune system, expressing them in higher amounts than other cells.In fact, breaking down ATP to adenosine with those two enzymes is thought to be one of the main ways in which Tregs suppress the immune system.
[source1][source2][source3]
So the three possibilities are:
1. Not enough Tregs positive in CD39 and CD73 are being produced.
Not all Tregs express CD39, only a specific CD45RO+CCR6+ effector/memory-like subset of them, also called TREM. Interesting observations have been made in multiple sclerosis, where the overall number of Tregs was not much different from controls, but the amount of Tregs positive in CD39 was much lower in MS patients.
[source] It is possible that a similar thing is happening in ME/CFS, perhaps by a different mechanism.
2. Not enough CD39+ Tregs are being activated. Tregs, like any other T cells, have a T Cell Receptor (TCR) which upon stimulation activates the cell to its primary function. It has been observed in mice, that Tregs exhibit CD39 activity only when activated.
[source] It probably works similarly in humans. As for when Tregs become activated, that is where the data starts being non-conclusive
[source], so I'll leave it at that.
This could also lead to a lowered number of them, as Tregs highly express the P2X7 receptor, and are very sensitive to high concentrations of eATP. Without the protection of CD39-mediated ATP hydrolysis, they easily die, or their suppresive capabilites are inhibited.
[source]
3. Direct obstruction of CD39 activity.
This third option is the most interesting to me, as it ties some stuff together, but I also don't know if this is possible the way I think it is.
Let's go back for a moment to Ron's impedance assay. The plasma switch results indicated that it is something in the blood that is making the cells behave differently than healthy ones. After reading all this you might think that this something is eATP, but it is most probably not. First, I have seen no publications indicating possible endocrine ATP signaling, only short-range paracrine (to neighboring cells) and autocrine (to self). Second, the filtration results showed that this factor in the plasma is larger than 10kDa, which ATP is not.
But then you also have the result that adding suramin "greatly reduced" the sickly response. Suramin is a non-selective P2 antagonist, blocking many of the P2X and P2Y receptors, to varying degrees. So how can a similar response be achieved by blocking ATP signaling as by filtering out large stuff from the plasma?
The answer can be an anti-CD39 antibody. This antibody would be present in ME/CFS blood and would inhibit ATP breakdown, increasing the activation of P2 receptors, opening of Panx1 channels, resulting in loss of ATP from the cell, constant sort-of-inflammation and many of our symptoms. When it is filtered out, CD39 can work and hydrolyze ATP, returning the cells to normal. When P2 receptors are blocked directly, the cells also return to normal.
This would explain why B cell depletion with Rituximab helps (activated B cells produce antibodies) and why TGF-β was one of the only two elevated cytokines in Montoya's and Mark Davis's findings
[source]; TGF- β is one of the other ways in which Tregs suppresses the immune system. It might be so, that the Tregs are working hard to resolve the inflammation, but cannot do it fully because CD39 is blocked by an antibody.
This is an idea that the immune system is basically attacking its own stop button. Or rather a part of it.
There are two problems with this hypothesis, and I have no idea if they can be resolved. First, ME/CFS plasma makes healthy cells behave like ME/CFS cells. In this hypothesis the factor this plasma introduces is the anti-CD39 antibody. But for this antibody to do anything at all to the cell, eATP must be already present. And the cells are healthy, from a human without an active infection. Do certain levels of ATP release occur at all times enough for the blockage of CD39 to make a difference? Or was some eATP floating around in the ME/CFS plasma? I don't know, but it seems like a stretch.
The second problem is the humoral immunity profiling ME/CFS study done by Lombardi et al.
[source] They used random peptide microarrays to check all the antibodies in the blood and what peptides they bind to. The aminoacid pattern they identified as a common thing was GVALSG. I checked, it doesn't match the sequence of CD39 at all. Now, I don't know the technical limitations of that study and the possibility that it would miss a key antibody.
Recombinant (synthetic) anti-CD39 antibodies are a thing though, so maybe this can be tested in the impedance assay.
There is another similar possibility that doesn't involve CD39 at all, and that is that the P2 receptors are being activated by some other agonist, not ATP, and that agonist also is larger than 10kDa and is our mysterious plasma factor. Maybe it could be an antibody. Just in case, I compared the sequences of all seven P2X receptors to the GVALSG pattern, and the best I got was 3 aminoacids in a pattern. I don't know much about antibody binding, but I find it hard to imagine a sequence of 3 would make for any meaningful binding. As for a non-antibody protein capable of P2X agonism, the only two I've found so far (mostly by accident, as I almost haven't touched this angle yet) are biglycan
[source] (43kDa) and Serum Amyloid A
[source] (11-14kDa, depending on subtype). (This is a super-long stretch, and probably not related, but I thought I would also mention it - particles of the NLRP3 inflammasome, which is activated in macrophages by eATP to release IL-1β, can also be released extracellularly, and probably are also a DAMP.
[source])
Another possibility that I haven't explored in detail, is that the mysterious blood factor upregulates P2 receptor expression in cells, making them more vulnerable to eATP signaling. Alan Light did find upregulated P2X4 and P2X5 expression in ME/CFS patients post-exercise.
[source1][source2] This would have the same problem as the anti-CD39 hypothesis in the sense that it doesn't seem to explain healthy cells becoming sickly in ME/CFS plasma.
So what about other inhibitory mechanisms of purinergic signaling, other than ecto-nucleotidases? The only other thing that I found, were some observations that P2X7 activation can inhibit Panx1-mediated ATP release.
[source] Yes, this is the reverse of what I said at the beginning. There were two studies that reported this, one on murine cells, other on human HEK293 cells. This is a cell line cultured from embryonic kidney cells, often used in research because it's easy to modify them to do what you want, so they have different properties based on how you "bake them". In this case, Panx1 was artificially introduced to those cells through transfection.
Those observations raise two possibilities. First, that depending on the cell type, P2X7 activation might open or close Panx1 channels. The cell types in which P2X7 activation has been observed as leading to Panx1 channel opening are macrophages
[source], astrocytes
[source], and enteric neurons
[source]. (And maybe more, I just haven't found the papers about it.) The P2X7-mediated Panx1 activation seems to be widely accepted in literature, but citations about it are often not present. It is very possible that in some cells P2X7 activation opens Panx1 and in other types closes them.
The second, more interesting possibility, is that there is some other factor, which decides if P2X7 activation in a cell opens or closes Panx1 channels. A distinction on P2X7 splice variants might be it.
[source] Or it might be some yet undiscovered thing, possibly our mysterious blood factor, or something tied to it biochemically. We don't really know.
On this anticlimactic note, I want to conclude Part 1.
Possible future ponderings
What about the complex approach that I mentioned earlier? The complex approach is to understand exactly how does resolution of inflammation work. How does antibody synthesis stop. When are Tregs activated and when are they suppressed. How do Panx1 channels close. How does this all tie together in a system that "knows" when pathogen extermination is over and when it's time to suppress the inflammatory response. Then we can try to find out what in that sequence might have gone wrong in ME/CFS. My thoughts on this will come in Part 2, if I don't get overwhelmed by it. Probably will take me quite a long while.
Now, in the title, I put "in pursuit of a unified ME/CFS theory". That's the goal, but I'm nowhere near it. I feel like before we can talk about even a speculatory unified theory of ME/CFS, four other areas need to be addressed in detail.
First is the connection to the gut. In the gut, weird things happen with the immune system. For example, intestinal CD8+ T cells have higher P2X7 receptor expression than CD8+ (cytotoxic) T cells found in other places of the body. This causes them to be very sensitive to eATP, in a manner similar to Tregs. I wonder what's their CD39 expression like. The digestive system also has its own variation of Tregs to not allow rampant immune reactions to the food passing through it, and to the natural intestinal bacteria. Could it be possible that reduced CD39 activity causes more pathological immune response in the gut, resulting in our digestive symptoms and destroying good bacteria? Maybe. Could it also tie back into why the CD39 activity is lower, in a feedback loop? I don't know. I need to read more.
Second is the connection to the brain. In the nervous system eATP is a neurotransmitter. It also activates microglia, which are basically the nervous system's macrophages. Jarred Younger's research points to microglia activation in ME/CFS and it could also explain some, or all, of our neurological symptoms, as well as the common dysautonomia. There could also be a feedback loop in here.
Third is the connection to exercise and PEM. The exacerbation of symptoms after exercise is a very distinct, hallmark symptom of ME/CFS and is the main thing that makes ME/CFS such a disabling condition. I have yet to see a plausible biochemical model of PEM, and I believe this is a compulsory requirement for any general theory of ME/CFS. The delayed nature of PEM and the fact that mental exercise can also cause it, can be important clues to what it is biochemically.
Fourth is the intracellular stuff. This is the hard part, because of how complex it is, as there are hundreds/thousands of proteins involved in the functioning of any cell. But if we are to tie this to PDH inhibition, glycolysis inhibition, and to the metabolomics findings, we need to understand what does purinergic signaling do inside the cell, how it impacts the cellular metabolism. The impedance assay results I think show that the signal difference is due to insufficient ATP inside the cell to pump out the salt. This is the only thing injecting ATP or pyruvate into the cell, and adding suramin to the plasma have in common - they increase the ATP in the cell. The question is - is the insuffucient ATP due to the loss through pannexin channels, or due to impaired ATP production, or both? If both, are they connected? In which direction? I have some ideas about how to answer those questions with the impedance assay, but I would have to know more about the specifics of it first, because I might have some incorrect assumptions here (Ron, if you're reading this, can I ask you a few questions about the assay?)
Theorizing about intracellular stuff is even more difficult because it will differ depending on cell type, but a common core is very possible. Dr Naviaux certainly seems to think this is the case, because his Cell Danger Response theory is exactly this. I still don't understand many things about it, like why are the metabolomics results in ME/CFS the reverse of an acute CDR. But it certainly makes sense in a lot of ways.
So, as for future continuations of this. The gut and brain connections I will probably explore in detail when I have the time and energy for it, and write part 3 and part 4 about it. The PEM aspect is more difficult to search for existing publications. The best way would be for a specialist in exercise physiology to look over all of the indicated pathways, and tell if something rings a bell as something they know as changing with physical exercise. (If any of you know good papers about cell-to-cell communication during exercise, send them my way) And the intracellular thing... .___. I don't wanna do it. That stuff is hard. But if someone else wants to try, I want to present to them
the most fucking confusing paper I have yet found. It states that CD39 (which is outside the cells), makes the cells have less ATP *inside*. Yes, this is exactly opposite to everything I said here. Further, it states that Tregs have lower intracellular ATP that Th cells, and those low levels make cyclophosphamide able to selectively kill Tregs, sparing other T cells. Yes, cyclophosphamide is the drug currently being trialed in Norway (along with Rituximab) as a treatment for ME/CFS based on some case studies showing that it helps. Now, this specific confusion I can resolve, it's just dose-dependent. Tregs are more sensitive to cyclophosphamide, so it only kills them, boosting the immune response (used in cancer), but high-dose cyclo kills all T cells, suppressing the immune response (used in autoimmune disease). As for why the rest makes no sense - I have no idea. It can be an error in the methodology of the study, or the results might be correct and tie into our illness in a clever way that I'm too stupid to understand. Or it might be totally unrelated.
Implications for treatment
To close things of, I wanted to touch a bit on treatment, albeit very carefully. Please don't take my words as medical advice.
If rampant eATP signaling is at the core of ME/CFS, then suramin by blocking it could indeed by very beneficial. However. If the reason for this is impaired ATP hydrolysis through ecto-nucleodidases, there is another thing to consider. Suramin, other than its P2 antagonism, was also shown to inhibit ecto-nucleotidases.
[source] The question is if this effect is less or more potent than the P2 antagonism. I would imagine less, based on how the autism trial went. But there is also the issue of adenosine not being produced and not activating the P1 anti-inflammatory receptors. Now, I'm not saying that suramin is a bad idea and we shouldn't try it in ME/CFS. My very uninformed guess is that overall the effect would still be positive. But it might not be. So I just wanted to point this out.
There's also couple other interesting things I found in regard to treatment.
Firstly, a couple of years ago, the AstraZeneca pharmaceutical company filed a patent for AZD9056, a potent, orally bioavailable, selective P2X7 inhibitor. It was to be used in rheumatoid arthritis. The drug was tolerated well, but failed to bring any symptomatic change in a phase IIb clinical trial, so it never reached the market. I have no idea if they would be interested in doing this, but what if AstraZeneca could pay for an ME/CFS clinical trial of AZD9056?
Secondly, it seems that the food dye FD&C Blue No. 1, also known as Brilliant Blue FCF, or E133, is a Panx1 inhibitor with neutral effect on P2X7.
[source] I don't really know what dose would be needed for it to have any meaningful effect, and if that dose is still known to be safe, but it might be possible to calculate this if we can somehow find out its bioavailability. Just please don't go eating some dangerous amount of this food dye, okay? I'm kinda wary of sharing this last thing in an open forum, but I'm gonna trust you guys to be responsible.
Thirdly, there are some herbs known to be P2 inhibitors.
@Jesse2233 beat me to the punch in making
a thread about it, as he usually does. There are more than just the two he mentioned though. I might write a post about them after I look more into it.
Thanks for reading.