'Nuggets' from the PACE trial full paper and webappendix.
These are my interpretations and I suggest you check against the papers to determine for yourself.
For brevity I have not included the details. I understand the papers are now available in the library.
Chalder Fatigue Questionnaire
One of the primary outcome measures if fatigue as measured by the Chalder fatigue questionnaire (CFQ). The paper states that entry criteria to PACE included being assessed as having a CFQ score of 6 or above (0 to maximum of 11) using the bimodal scoring system. The paper also states that in the outcomes analysis, Lickert scoring (0,1,2,3; range 0-33, lowest = least fatigue) was substituted for bi-modal scoring. Self rated outcomes were assessed at 12, 24 and 52 weeks.
Amongst the criticisms of the CFQ is that, along with other commonly used fatigue scales, it is prone to ceiling effects :
http://www.springerlink.com/content/p8g2h76327n08151/
“Extreme scoring occurred on a large number of the items for all three recommended fatigue rating scales across several studies. The percentage of items with the maximum score exceeded 40% in several cases. The amount of extreme scoring for a certain scale varied from one study to another, which suggests heterogeneity in the selected subjects across studies.
Because all three instruments easily reach the extreme ends of their scales on a large number of the individual items, they do not accurately represent the severe fatigue that is characteristic for CFS. This should lead to serious questions about the validity and suitability of the Checklist Individual Strength, the Chalder Fatigue Scale, and the Krupp Fatigue Severity Scale for evaluating fatigue in CFS research.”
In this respect, the same author states that the bimodal scoring version of the CFQ is even more prone to ceiling effects.
The practical outcome of this recognised problem is that many respondents will score at the scale maximum at outset and the instrument will be unable to detect any exacerbation in fatigue (that is an adverse effect).
In addition if the bimodal scoring version of the CFQ is more prone to ceiling effects (that is extreme scores) than the continuous scoring version then it logically follows that the bimodal version is prone to exaggerating the level of reported fatigue.
It is highly likely, if not unavoidable, that rating the same subjects, at the same point in time using the continuous scoring version compared to bimodal scoring would result in a lower overall fatigue score across the board.
Over two points in time, you would likewise expect that patients fatigue scores assessed at baseline using bimodal scoring would rate fatigue higher than the same patients ratings at the first outcome point, at 12 weeks, using continuous scoring.
In other words self rated fatigue would be seen to decline between the two points solely as a function of the revised scoring system regardless of intervention.
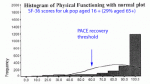
Table 3 and figure 2 of the PACE paper show CFQ results for each treatment arm and participants grouped by diagnostic label.
It can be seen that, regardless of treatment arm or diagnostic label, fatigue scores in all cases fell by between 4 and 6 points from baseline to 12 weeks and this magnitude of change was not seen for any group between 12 and 24 weeks or 12 and 52 weeks.
In conclusion, the majority of the improvement in fatigue scores for all treatment arms occurred between baseline and 12 weeks and was likely due to post hoc changes made to the scoring system used.
More to follow.