Manuel
Senior Member
- Messages
- 108
Good afternoon,
I am Manuel Ruiz, medical student in Spain and I am sick with CFS/ME. Together with my girlfriend Rosario, graduated in Medicine from the Complutense University of Madrid, we contacted one of the best research centers in Spain, CIMA, to carry out the project described below.
Currently I have seen quite a few lymphocyte typages that have provided me with CFS/ME patients. They could be grouped into two main groups:
-In the first group they present reduced levels of total t lymphocytes (CD4, CD8, activated t lymphocytes) that indicate the presence of an immunodeficiency of T lymphocytes.
-In the second group the patients have total t lymphocytes (CD4, CD8) and immunoglobulins in normal values. But this does not mean that there cannot be some type of cell immunodeficiency. The analysis of activated CD3+ HLA-DR+ and CD3+ CD4+ HLA-DR+ T lymphocytes is used to see if there is indirectly a decrease in the MHC class II antigenic presentation, since CD4 T lymphocytes are activated through this antigenic presentation made by B lymphocytes (antigen-presenting cells) in the secondary lymphoid organs. Therefore, if there is a decrease in the expression of class II histocompatibility molecules in B lymphocytes, they would not present antigens to CD4 T lymphocytes and these would not be activated. For any T-lymphocyte to be able to perform its functions and become an effector lymphocyte, it is necessary for them to be activated. That is to say, the values of the activated CD4+ HLA-DR+ T lymphocytes indicate indirectly if there is any problem in the class II MHC antigenic presentation made by the B lymphocytes.
There is a congenital immunodeficiency called class II MHC deficiency in antigen-presenting cells, which is associated with a severe decrease in CD4+ (activated) T lymphocytes. This absence of cooperating T lymphocytes (CD4+) causes a deficiency in humoral response (the low number of CD4+ T lymphocytes causes a defect in collaboration between T and B lymphocytes) and cellular response (due to the intrinsic defect in the number of CD4+ T lymphocytes). Patients suffer repeated infections, particularly of the digestive tract. The genetic defect of this severe immunodeficiency is found in several proteins regulating the transcription of HLA class II genes.1
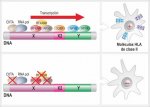
Figure (photo attached): Regulation of class II HLA genes in normal individuals (above) and in patients with class II HLA deficiency (below).1
The following are the ESID (European Society for Immunodeficiencies) diagnostic criteria for this congenital immunodeficiency:2
A. Definitive diagnosis: if both criteria are met:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Mutation in one of the genes: CIITA, RFX-B, RFX-5 or RFX-AP.
B. Probable diagnosis: if all four criteria are met:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Failure to thrive, opportunistic infections or persistent viral infections.
• Normal number of T and B cells.
• Normal proliferative responses to mitogens.
C. Possible diagnosis: the first of the criteria plus at least one of the following:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Hypogammaglobulinemia.
• Normal mitogen responses but absent T cell proliferation to antigens.
• Normal number of T and B cells.
• Reduced number of CD4+ cells.
• Failure of mononuclear cells to stimulate a mixed lymphocyte culture.
The four genetic disorders that give rise to this congenital immunodeficiency are clinically indistinguishable. In most cases, there is no Class II expression. However, in others, the intensity of expression of MHC-II molecules may be as high as 5% of normal. Patients with higher expression tend to have a milder course of disease. These patients may survive beyond early childhood.3
We believe that the difference between this congenital immunodeficiency and that found in the second group of CFS/ME is in the number of antigen-presenting cells that have class II MHC deficiency. In the CFS/ME group it would only occur in those cells infected (mostly B lymphocytes) with an intracellular pathogen and in congenital immunodeficiency is in all antigen-presenting cells.
There are several intracellular pathogens that reduce the MHC class II antigenic presentation as an evasion mechanism of the immune system. The best known is the Epstein Barr virus, where it has been demonstrated that the latent protein LMP2A mediated the reduction of CIITA levels by decreasing the expression of PU.1 and E47 in B cells.4 Others such as human cytomegalovirus, human parainfluenza virus type 3, and varicella zoster virus suppress the IFN-γ-induced expression of class II MHC through inhibition of JAK-STAT activation and the transcription route activator, resulting in a reduction of CIITA expression.4 This may confirm the infectious origin of SFC/ME.
Both groups of CFS/ME have a type of immunodeficiency and it appears that those with a total T lymphocyte immunodeficiency (not just activated) are in a more advanced stage of the disease, and those with only decreased activated t lymphocytes are in an early stage. Both justify the recurrent infections that these patients present and the chronic fatigue that they present.
What would be achieved if this hypothesis were met?
-1. A diagnosis of immunodeficiency. We would no longer be invisible to health care.
-2. Access treatments for this type of immunodeficiency.
-3. Begin to develop new specific treatments that eliminate cells with latent intracellular pathogens (such as the Epstein Barr virus).
After everything described, I contacted the research team of Dr. Bruno Paiva (https://cima.unav.edu/investigacion/plataformas/citometria/equipo/bruno-paiva) of CIMA of the University of Navarra.
This is the link of crowdfunding:https://helpify.es/comunidades/todo-por-la-causa-del-sindrome-de-la-fatiga-cronica/
I hope you will help us with outreach and fundraising.
I will try to solve all the doubts that I can.
Greetings
Bibliography.
1. Regueiro González J.R., López Larrea C., González Rodriguez S. y Martínez Naves E. Inmunología: Biología y patología del sistema inmunitario. 4ª edición. Editorial Médica Panamerica, 2010.
2. Serrano Martín MªM. , et all. Déficit de expresión de moléculas de clase II del complejo mayor de histocompatibilidad. Anales de pediatría. Marzo 2007. Vol: 66, nº3 pag:227-339. Available in:
http://www.analesdepediatria.org/es-deficit-expresion-moleculas-clase-ii-articulo-13099694
3. MHC class II deficiency diagnostic criteria. European Society for Inmunodeficiencies. Available in:
https://esid.org/Working-Parties/Clinical-Working-Party/Resources/Diagnostic-criteria-for-PID2#Q10
4. Jiun-Han Lin, Ju-Yin Lin, Ya-Ching Chou, Mei-Ru Chen, Te-Huei Yeh, Chung-Wu Lin, Sue-Jane Lin and Ching-Hwa Tsai. Epstein-Barr virus LMP2A suppresses MHC class II expression by regulating the B-cell transcription factors E47 and PU.1. American Society of Hematology. April 2, 2015. Col. 125 no. 14 2228-2238. Available in: http://www.bloodjournal.org/content/125/14/2228/tab-figures-only?sso-checked=true
I am Manuel Ruiz, medical student in Spain and I am sick with CFS/ME. Together with my girlfriend Rosario, graduated in Medicine from the Complutense University of Madrid, we contacted one of the best research centers in Spain, CIMA, to carry out the project described below.
Currently I have seen quite a few lymphocyte typages that have provided me with CFS/ME patients. They could be grouped into two main groups:
-In the first group they present reduced levels of total t lymphocytes (CD4, CD8, activated t lymphocytes) that indicate the presence of an immunodeficiency of T lymphocytes.
-In the second group the patients have total t lymphocytes (CD4, CD8) and immunoglobulins in normal values. But this does not mean that there cannot be some type of cell immunodeficiency. The analysis of activated CD3+ HLA-DR+ and CD3+ CD4+ HLA-DR+ T lymphocytes is used to see if there is indirectly a decrease in the MHC class II antigenic presentation, since CD4 T lymphocytes are activated through this antigenic presentation made by B lymphocytes (antigen-presenting cells) in the secondary lymphoid organs. Therefore, if there is a decrease in the expression of class II histocompatibility molecules in B lymphocytes, they would not present antigens to CD4 T lymphocytes and these would not be activated. For any T-lymphocyte to be able to perform its functions and become an effector lymphocyte, it is necessary for them to be activated. That is to say, the values of the activated CD4+ HLA-DR+ T lymphocytes indicate indirectly if there is any problem in the class II MHC antigenic presentation made by the B lymphocytes.
There is a congenital immunodeficiency called class II MHC deficiency in antigen-presenting cells, which is associated with a severe decrease in CD4+ (activated) T lymphocytes. This absence of cooperating T lymphocytes (CD4+) causes a deficiency in humoral response (the low number of CD4+ T lymphocytes causes a defect in collaboration between T and B lymphocytes) and cellular response (due to the intrinsic defect in the number of CD4+ T lymphocytes). Patients suffer repeated infections, particularly of the digestive tract. The genetic defect of this severe immunodeficiency is found in several proteins regulating the transcription of HLA class II genes.1
Figure (photo attached): Regulation of class II HLA genes in normal individuals (above) and in patients with class II HLA deficiency (below).1
The following are the ESID (European Society for Immunodeficiencies) diagnostic criteria for this congenital immunodeficiency:2
A. Definitive diagnosis: if both criteria are met:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Mutation in one of the genes: CIITA, RFX-B, RFX-5 or RFX-AP.
B. Probable diagnosis: if all four criteria are met:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Failure to thrive, opportunistic infections or persistent viral infections.
• Normal number of T and B cells.
• Normal proliferative responses to mitogens.
C. Possible diagnosis: the first of the criteria plus at least one of the following:
• Deficit expression (<5%) of MHC-II molecules in B lymphocytes or monocytes.
• Hypogammaglobulinemia.
• Normal mitogen responses but absent T cell proliferation to antigens.
• Normal number of T and B cells.
• Reduced number of CD4+ cells.
• Failure of mononuclear cells to stimulate a mixed lymphocyte culture.
The four genetic disorders that give rise to this congenital immunodeficiency are clinically indistinguishable. In most cases, there is no Class II expression. However, in others, the intensity of expression of MHC-II molecules may be as high as 5% of normal. Patients with higher expression tend to have a milder course of disease. These patients may survive beyond early childhood.3
We believe that the difference between this congenital immunodeficiency and that found in the second group of CFS/ME is in the number of antigen-presenting cells that have class II MHC deficiency. In the CFS/ME group it would only occur in those cells infected (mostly B lymphocytes) with an intracellular pathogen and in congenital immunodeficiency is in all antigen-presenting cells.
There are several intracellular pathogens that reduce the MHC class II antigenic presentation as an evasion mechanism of the immune system. The best known is the Epstein Barr virus, where it has been demonstrated that the latent protein LMP2A mediated the reduction of CIITA levels by decreasing the expression of PU.1 and E47 in B cells.4 Others such as human cytomegalovirus, human parainfluenza virus type 3, and varicella zoster virus suppress the IFN-γ-induced expression of class II MHC through inhibition of JAK-STAT activation and the transcription route activator, resulting in a reduction of CIITA expression.4 This may confirm the infectious origin of SFC/ME.
Both groups of CFS/ME have a type of immunodeficiency and it appears that those with a total T lymphocyte immunodeficiency (not just activated) are in a more advanced stage of the disease, and those with only decreased activated t lymphocytes are in an early stage. Both justify the recurrent infections that these patients present and the chronic fatigue that they present.
What would be achieved if this hypothesis were met?
-1. A diagnosis of immunodeficiency. We would no longer be invisible to health care.
-2. Access treatments for this type of immunodeficiency.
-3. Begin to develop new specific treatments that eliminate cells with latent intracellular pathogens (such as the Epstein Barr virus).
After everything described, I contacted the research team of Dr. Bruno Paiva (https://cima.unav.edu/investigacion/plataformas/citometria/equipo/bruno-paiva) of CIMA of the University of Navarra.
This is the link of crowdfunding:https://helpify.es/comunidades/todo-por-la-causa-del-sindrome-de-la-fatiga-cronica/
I hope you will help us with outreach and fundraising.
I will try to solve all the doubts that I can.
Greetings
Bibliography.
1. Regueiro González J.R., López Larrea C., González Rodriguez S. y Martínez Naves E. Inmunología: Biología y patología del sistema inmunitario. 4ª edición. Editorial Médica Panamerica, 2010.
2. Serrano Martín MªM. , et all. Déficit de expresión de moléculas de clase II del complejo mayor de histocompatibilidad. Anales de pediatría. Marzo 2007. Vol: 66, nº3 pag:227-339. Available in:
http://www.analesdepediatria.org/es-deficit-expresion-moleculas-clase-ii-articulo-13099694
3. MHC class II deficiency diagnostic criteria. European Society for Inmunodeficiencies. Available in:
https://esid.org/Working-Parties/Clinical-Working-Party/Resources/Diagnostic-criteria-for-PID2#Q10
4. Jiun-Han Lin, Ju-Yin Lin, Ya-Ching Chou, Mei-Ru Chen, Te-Huei Yeh, Chung-Wu Lin, Sue-Jane Lin and Ching-Hwa Tsai. Epstein-Barr virus LMP2A suppresses MHC class II expression by regulating the B-cell transcription factors E47 and PU.1. American Society of Hematology. April 2, 2015. Col. 125 no. 14 2228-2238. Available in: http://www.bloodjournal.org/content/125/14/2228/tab-figures-only?sso-checked=true