I have had moderate ME for nearly three years and I have tried alot of treatments which I list in my introduction post.
I am lucky that I tolerate most medicines and vaccines well. I do not get infections often or badly and I live in the UK where I can rely on free healthcare if something goes wrong.
I have done my best to fact check, but please do no trust anything I say when it comes to this very serious drug.
Intravenous Cyclophosphamide in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. An Open-Label Phase II Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7201056/
I am aware this study has been discussed before, but I am adding my own summary and opinions to explain why I am doing it.
What was the response rate
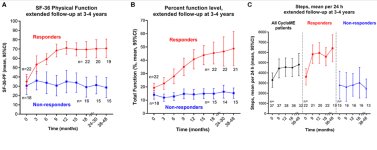
21/40 patients, (52.5%) reported at least a slight improvement in their condition at end of four year follow-up. Some of these saw early improvement and partial relapse. Others had a slow and steady improvement. At baseline, only two of the patients had part-time work participation. During follow-up, at least nine patients returned to either part-time of full-time work or studies.
The researchers announced at the 2023 charite conference that (7/40 17.5%) patients reported near full recovery at a six year follow up. For comparison out of the placebo group for the rituximab study done by the same researchers only 8% reached full recovery in this time which could be regarded as an unblinded control group.
https://meassociation.org.uk/wp-con...E-MECFS-RESEARCH-CONFERENCE-2023-05.06.23.pdf
Is it possible to predict who will respond?
The presence of either of the two HLA risk alleles, (HLA-DQB1*03:03 and HLA-C*07:04) (30), was predictive for response to cyclophosphamide. 83% response rate for those with vs. 43% response for those without. I have not got tested because in the event it was negative I would still take a 43% chance.
You can get tested for HLA alleles at www.cd-genomics.com
Why I think these results could be improved
If more patients had completed the treatment it is likely the response rate would be slightly higher. I believe that fewer patients would drop out in future because the success of this study and the expectation of a very slow response would give them more motivation to continue. 38/40 (95%) did at least four doses which suggests that these doses are tolerable. 34/40 (85%) did at least five doses. 31/40 (77.5%) did all six doses.
The dose could be lowered for the patients who are considering stopping treatment.
It is possible that giving an extra one or two doses to the responders a year later might improve the depth of response.
Why wasn’t the trial blinded?
It is impossible to blind cyclophosphamide because of the side effects.
Why I think this was unlikely to be a placebo effect
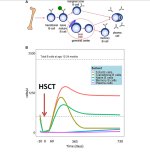
I can’t be sure but it seems unlikely because of the exceptionally slow response time and in most responders a lasting improvement. The researchers originally intended a two year observation period, but extended it to four years when they realised some patients were still improving. They did an extended follow up at six years and some patients had continued to improve past four years.
Why there will not be another cyclophosphamide for ME trial
Despite the promise shown by this trial the researchers decided to start again with a six patient pilot trial of daratumumab. This drug is safer and less unpleasant but at present unaffordable for most patients.
Dr Fluge said this is largely because of concerns about cyclophosphamides effect on fertility. Few of us feel able to provide or care for more children and many would gladly risk our fertility for a chance of a better life. There is a drug that when taken before cyclophosphamide can reduce the risk to female fertility. Because there are unlikely to be any more trials then a very promising and affordable treatment could be lost forever.
How did the study participants tolerate the drug
“In general, the toxicity to cyclophosphamide infusions in ME/CFS patients was moderate, and there were few serious adverse events and no registered hematological toxicity. The most common side effects were nausea and general malaise lasting for 1–2 weeks after each infusion. ME/CFS patients reported more nausea and discomfort after cyclophosphamide than cancer patients typically do at similar doses, in line with the generally low stress tolerance and sensitivity to drugs reported by many patients”
What are the risks?
There is too much to be said about this for me to try and summarise. After a reading a quick summary like webmd the risks sound so bad that most people dismiss it immediately. A deeper read says that the risks are serious, but significantly lower for the relatively low doses and lifetime cumulative doses used in the this study.
None of the 40 study participants reported long term worsening, but it is a small sample. There are ME patients who have reported long term worsening after much less serious treatments (including LDN) so it is likely it would happen to someone.
What doses did they use in the study and how do they compare to doses used for cancer?
The cyclophosphamide for ME trial used a first dose of 600mg/m2 followed by five monthly doses at 700mg/m2. For a 70kg/155lb person this equates to a first dose of about 1080mg followed by 5 x 1260mg doses. Total lifetime dose of about 7.4G.
For comparison cancer doses of cyclophosphamide are as high as 50mg/kg which equates to roughly 3.5g administered over 2-5 days. The immunoablative protocol for MS is 200mg/kg (14G) over four days.
The maximum recommended lifetime dose of cyclophosphamide depends on the disease. To pick one example the lifetime dose for autoimmune vasculitis should not exceed 25g (NHS).
Is it difficult to learn to give yourself an IV infusion
Anyone can learn, but it takes practice.
Can it be taken orally?
Some doctors prescribe a pulsed oral dose of 500mg although this is less common practice.
Where am I getting it
Indiamart
Am I worried about doing it without a doctor?
Controversial opinion, I do not believe the risk is significantly higher. The reasons I believe this are
Is it plausible that a higher number of lower doses might work for ME?
It is plausible because lower doses work for autoimmune diseases. The study used the typical doses for autoimmune diseases like lupus at the time of study design. Since then some doctors have moved towards using more frequent lower doses of cyclophosphamide to treat these diseases (500mg every two weeeks). Although there is no consensus about whether lower doses are equally effective, they are safer and less unpleasant so some might see it as a better risk/reward ratio.
I am lucky that I tolerate most medicines and vaccines well. I do not get infections often or badly and I live in the UK where I can rely on free healthcare if something goes wrong.
I have done my best to fact check, but please do no trust anything I say when it comes to this very serious drug.
Intravenous Cyclophosphamide in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. An Open-Label Phase II Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7201056/
I am aware this study has been discussed before, but I am adding my own summary and opinions to explain why I am doing it.
What was the response rate
21/40 patients, (52.5%) reported at least a slight improvement in their condition at end of four year follow-up. Some of these saw early improvement and partial relapse. Others had a slow and steady improvement. At baseline, only two of the patients had part-time work participation. During follow-up, at least nine patients returned to either part-time of full-time work or studies.
The researchers announced at the 2023 charite conference that (7/40 17.5%) patients reported near full recovery at a six year follow up. For comparison out of the placebo group for the rituximab study done by the same researchers only 8% reached full recovery in this time which could be regarded as an unblinded control group.
https://meassociation.org.uk/wp-con...E-MECFS-RESEARCH-CONFERENCE-2023-05.06.23.pdf
Is it possible to predict who will respond?
The presence of either of the two HLA risk alleles, (HLA-DQB1*03:03 and HLA-C*07:04) (30), was predictive for response to cyclophosphamide. 83% response rate for those with vs. 43% response for those without. I have not got tested because in the event it was negative I would still take a 43% chance.
You can get tested for HLA alleles at www.cd-genomics.com
Why I think these results could be improved
If more patients had completed the treatment it is likely the response rate would be slightly higher. I believe that fewer patients would drop out in future because the success of this study and the expectation of a very slow response would give them more motivation to continue. 38/40 (95%) did at least four doses which suggests that these doses are tolerable. 34/40 (85%) did at least five doses. 31/40 (77.5%) did all six doses.
The dose could be lowered for the patients who are considering stopping treatment.
It is possible that giving an extra one or two doses to the responders a year later might improve the depth of response.
Why wasn’t the trial blinded?
It is impossible to blind cyclophosphamide because of the side effects.
Why I think this was unlikely to be a placebo effect
I can’t be sure but it seems unlikely because of the exceptionally slow response time and in most responders a lasting improvement. The researchers originally intended a two year observation period, but extended it to four years when they realised some patients were still improving. They did an extended follow up at six years and some patients had continued to improve past four years.
Why there will not be another cyclophosphamide for ME trial
Despite the promise shown by this trial the researchers decided to start again with a six patient pilot trial of daratumumab. This drug is safer and less unpleasant but at present unaffordable for most patients.
Dr Fluge said this is largely because of concerns about cyclophosphamides effect on fertility. Few of us feel able to provide or care for more children and many would gladly risk our fertility for a chance of a better life. There is a drug that when taken before cyclophosphamide can reduce the risk to female fertility. Because there are unlikely to be any more trials then a very promising and affordable treatment could be lost forever.
How did the study participants tolerate the drug
“In general, the toxicity to cyclophosphamide infusions in ME/CFS patients was moderate, and there were few serious adverse events and no registered hematological toxicity. The most common side effects were nausea and general malaise lasting for 1–2 weeks after each infusion. ME/CFS patients reported more nausea and discomfort after cyclophosphamide than cancer patients typically do at similar doses, in line with the generally low stress tolerance and sensitivity to drugs reported by many patients”
What are the risks?
There is too much to be said about this for me to try and summarise. After a reading a quick summary like webmd the risks sound so bad that most people dismiss it immediately. A deeper read says that the risks are serious, but significantly lower for the relatively low doses and lifetime cumulative doses used in the this study.
None of the 40 study participants reported long term worsening, but it is a small sample. There are ME patients who have reported long term worsening after much less serious treatments (including LDN) so it is likely it would happen to someone.
What doses did they use in the study and how do they compare to doses used for cancer?
The cyclophosphamide for ME trial used a first dose of 600mg/m2 followed by five monthly doses at 700mg/m2. For a 70kg/155lb person this equates to a first dose of about 1080mg followed by 5 x 1260mg doses. Total lifetime dose of about 7.4G.
For comparison cancer doses of cyclophosphamide are as high as 50mg/kg which equates to roughly 3.5g administered over 2-5 days. The immunoablative protocol for MS is 200mg/kg (14G) over four days.
The maximum recommended lifetime dose of cyclophosphamide depends on the disease. To pick one example the lifetime dose for autoimmune vasculitis should not exceed 25g (NHS).
Is it difficult to learn to give yourself an IV infusion
Anyone can learn, but it takes practice.
Can it be taken orally?
Some doctors prescribe a pulsed oral dose of 500mg although this is less common practice.
Where am I getting it
Indiamart
Am I worried about doing it without a doctor?
Controversial opinion, I do not believe the risk is significantly higher. The reasons I believe this are
- I do not fit any of the contraindications.
- There is minimal risk for of an allergic reaction that would require immediate medical attention, but I have an adrenaline pen in case. I will be with two other people when I infuse.
- I am confident that I understand the risks and am capable of following the protocols.
- I have had all of the recommended vaccines.
- If an adverse event occurs I will go to a hospital and tell them what I have taken. Doctors would call me irresponsible, but treat me like any other patient.
Is it plausible that a higher number of lower doses might work for ME?
It is plausible because lower doses work for autoimmune diseases. The study used the typical doses for autoimmune diseases like lupus at the time of study design. Since then some doctors have moved towards using more frequent lower doses of cyclophosphamide to treat these diseases (500mg every two weeeks). Although there is no consensus about whether lower doses are equally effective, they are safer and less unpleasant so some might see it as a better risk/reward ratio.