If you google one carbon metabolism you might find more information which is not trying to cash in on trendy internet chatter (not that I think Chris Kesser is trying to do this - I agree with
@Basilico, he is usually a reliable source of information).
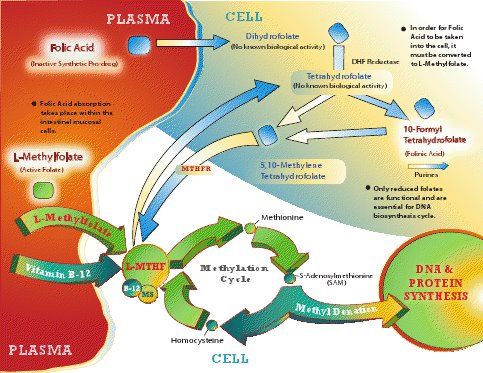
For this is what methylation is all about, the transfer of single carbon units (methyl groups) which are used in a wide variety of biosynthetic processes in the body.
At the heart of 1C transfer is the folate cycle which mediates three biosynthetic pathways,
de novo synthesis of purines, thymidylate synthesis and the remethylation of homocysteine to form methionine (a B12-dependant reaction).
Here is an illustration of these pathways which is derived from
this paper.
Methionine in turn is a precursor for the synthesis of
S-adenosylmethionine (AdoMet or SAM), a cofactor and methyl group donor for numerous methylation reactions, including the methylation of cytosine bases in DNA, histones, RNA, neurotransmitters, and other small molecules, phospholipids, and other proteins.
S-adenosylmethionine–dependent methylation reactions serve to regulate fundamental biological processes, including gene transcription, mRNA translation, cell signaling, protein localization, and the degradation of small molecules.
The primary source of one-carbons for cytoplasmic one-carbon metabolism comes from formate that is derived from mitochondrial one-carbon metabolism and the ultimate source of formate is the amino acid serine.
Here,
here and
here are university websites which illustrate the pathways in different ways. Sometimes people find different approaches more understandable.
Here is a paper which quantitates the relative importance of the different pathways, concluding that
Here is just old review which canvasses some of the ways these pathways are thought to be relevant to various health conditions. It is old but it does set things out clearly.
How does all this relate to ME/CFS?
We don't really know, though people have various theories, including Rich who you have already been directed to. Certainly many people on PR have reported benefit from folate/B12 supplements.
The recent work of
Naviaux might provide some clues about this. He found widespread metabolic derangement in ME/CFS patients, all of which were either directly regulated by redox or the availability of NADPH.
1C metabolism is directly related to this since transulfuration produces cysteine, the rate-limiting step in synthesis of the ultimate redox controller glutathione, while one of the folate cycle enzymes (MTHFD2L) is an important source of NADPH.