Indigophoton
Senior Member
- Messages
- 127
- Location
- UK
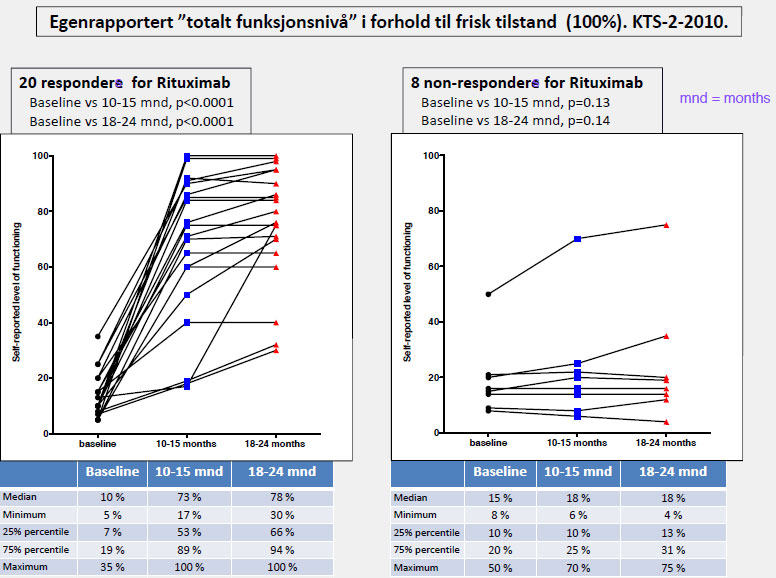
...it is a logical to try plasma exchange as initial therapy for this group, followed by Rituximab treatment...
@deleder2k Thanks for all the info you are posting and especially for this: as a very severely affected ME patient you have made my day (all the ifs and buts and maybes nothwithstanding)
Oystein Fluge seems to have so much common sense and compassion. Thank goodness there may be at least one or two individuals in the UK with the same:
What I think is encouraging is that at least one neurological colleague, expert in this field, is ready to take the issue of autoimmunity and immunotherapy in ME seriously and share thoughts.
Regarding,
I constantly ask myself why ME does not seem to be so 'urgent'? There are probably three answers. One is that some of the other conditions include movement disorders that prevent any normal activity or even standing up or sleep.
Hmm, well very severe ME often prevents standing up, sitting up, or even lifting one's arm or raising one's head up off the bed. It can prevent exposure to normal levels of sound and light, which is isolating in the extreme (no conversation, no sunshine, for example.) On top of the disability/loss of function, ME can also make one feel very ill most of the time, resulting in terribly poor quality of life. It's presumably just not obvious enough to others.
@Jonathan Edwards, is it possible that the preliminary work being done for the UK Rituximab trial would advance the search for a reliable marker for the disease, which might help stimulate research and medical activity, or is its aim solely to try to stratify patients into responders and non-responders for Rituximab treatment?