Another characteristic mentioned in the CDCP definition is a substantial reduction of the premorbid activity level (1), evidenced by clinical research (2,3). In addition to the reduced activity level compared to the premorbid level or to healthy controls. people with CFS display an abnormal activity pattern their lifestyle appears to be characterised by activity peaks and longer bouts of rest after activity (3). The latter is in line with a physiological study showing a delayed recovery from exercise in CFS patients (4). Clinical studies revealed that overly vigorous exercise (5, 6) or even a 30% increase in activity (7), frequently triggers a relapse. However, the cause of this post_exertional malaise and the altered or reduced activity level remains unclear.
It is hypothesised that nitric oxide (NO) is involved in these phenomena of altered and reduced activity level and of exercise intolerance. It is known that patients with CFS present elevated levels of blood NO (8). Excessive NO concentrations are detrimental for physiological functions via the derivative peroxynitrite (9). Peroxynitrite is not a free radical, but leaves the hallmarks of oxidation typical of free radicals (9). Furthermore, NO as a mediator of vasodilatation, is critical for basal blood flow across many organs. In consequence, elevated amounts of NO in CFS can cause hypotension (10). As previously suggested (11), this may explain part of the abnormal exercise response in CFS. NO-induced vasodilatation may limit the capacity of the human body to increase blood flow during physical activity, limiting activity performance in CFS-patients. In addition, physical activity further increases NO amounts and vasodilatation and thus hypotension (12-14). In CFS patients this effect could be aggravated by the already elevated amounts of NO, explaining the malaise and the delayed recovery after physical activity (4). Secondly, pathological overproduction of NO will decrease oxygen consumption (15) and increase anaerobic glycolysis (lactate production) by modulating mitochondrial respiration (16) and iron metabolism (17). Finally, NO could alter muscular morphology and function by oxidative damage of cell membranes (18), structural proteins such as actin (9), and DNA (19), causing muscle weakness, soreness and fatigue. All these mechanisms compromise exercise capacity and worsen physical activity responses.
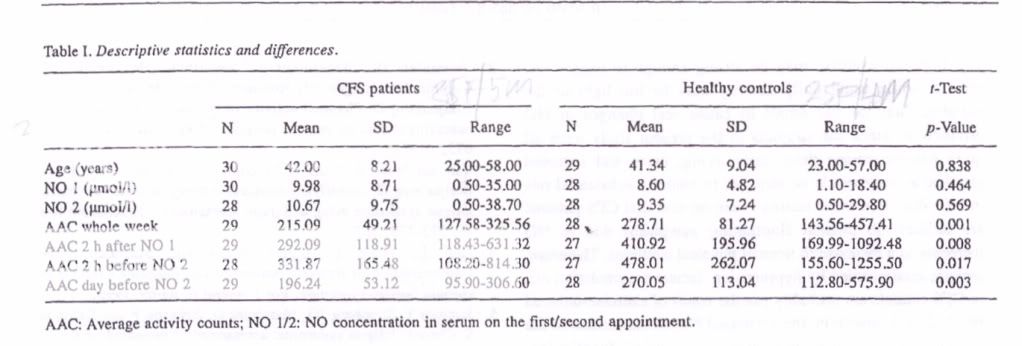
Therefore, it was hypothesised that NO plays an aetiological role in the reduced activity level and fluctuating symptom pattern in people with CFS. Either people with CFS are not capable of being physically active due to elevated NO amounts, or an activity peak could trigger NO release accompanied by post_exertional malaise.
In summary, the goal of this study was to investigate whether NO concentration in the serum was related to physical activity level in people with CFS and healthy sedentary controls.
References:
(spell-checker was going crazy here so I haven't gone through this but hopefully enough information for people to be able to recognise the study)
2 Vercoulen ill, Bazelmans B, Swaninck CM, Fennis IF,
Galama 3M, Jongen Fl, Jlommes 0, Van der Meer 3W and
Bleijenberg 0: Physical activity in chronic fatigue syndrome:
assessment and its role in fatigue.) Psychiatr Res 31: 661-
673. 1997.
3 Van der Werf 8, Prins 1, Vercouten 3, van der Meer I and Bieijenberg 0: Identifying physical activity patterns in chronic fatigue syndrome using actigraph assessment. .1 Psychosom Res
49: 373-379,2000.
4 Paul L, Wood L, Behan WMH and Maclaren WM:
Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur I Neurol 6: 63-69, 7996.
5 Jammes Y, Steinberg 10, Mambrini 0, Brgeon F and Delliaux
5: Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise.3 Intern Med 257: 299-310,2005.
6 Bazelmans B, B]ijenberg 0, Voeten MJM, van der Meer JWM
and Fotgering 14: Impact of a maximal exercise test on symptoms
and activity in chrnnic fatigue syndrome. I Psychnsnm Rca 59:
201-208, 2005.
7 Black CD, OConnor P3 and McCully KK: Increased daily physical activity and fatigue symptoms in chronic fatigue syndrome: Dynamic Med 4: 3-lI, 2005.
8 Kurup RK and ICurup PA: Hypothalamic digoxin, cerebraL chemical dominance and myalgic encephalomyelitis. mt I Neurosc 113: 683-701,2003.
9 Beckman IS and Koppeno] WH: Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugLy. Am .1 Physiol Cell Physiol 271: CI424-1437, 1996.
10 Kindig CA, McDonough P. Finley MR. Behnkc 81. Richardson TE, Marlin Di, Erickson HH and Poole DC: NO inhalation reduces pulmonary arterial pressure but not hemorrhage in maximal exercising horses. I AppI Physiol 91: 2674-2678, 2001.
II Nut 3, Dc Meirleir K. Mccus M, McGregor N and Engelebienne P: Chronic fatigue syndrome: intracellular immune deregulations as a possible etiology for abnormal exercise response. Mcd Hypothes 62: 759-765,2004.
12 Clroux 1, Kouame N, Nadeau A, Coulombe U and Lacourciere
Y: Aftereffects of exercise on regional and systemic hemodynamics in hypertension. Hypertension 19: 183-191, 1992.
13 Brown MD, Srinivasan M, Hogikyan RV, Dengel DR. Glickman
50, Galecki A and Supiano MA: Nitric oxide biomarkers increase during exercise-induced vasodilatioa in the forearm. Tnt Sports Mcd 21: 83-89, 2000,
14 Piepoli M. Coats AJS, Adamopoulos S. Bemardi L. Feng YB, Conway I and Sleight P: Persistent peripheral vasodilatation and sympathetic activity in hypotension after maximal exercise. JAppI Physiol 75: 1807-1814, 1993.
IS Shen W, Hintze TH and Wolin MS: Nitric oxide: an important signaling mechanism between vascular endotlielium and parenchymal cells in the regulation of oxygen consumption. Circulation 92: 3505-3512, 1995,
16 Tatsumi T, Matobo 5, ICawahara A, Keira N, Shiraishi I, Akashi IC, Kobara M,Thnaka T, Katamura M, Nakagawa C, Ohta B, Shirayama T, Takeda K. Asayama 1, Flits H and Nakagawa M:
Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. I Am Coil Cardiology 35: 1338-1346, 2000.
17 Qian ZM, Xao DS, KeY and Liao OK: Increased nitric oxide is
one of the causes of changes of iron melabolism in strenuously
exercised rats. Am J Physiol Regul Integr Comp Physiol 280,
R739-743. 2001.
18 Fulle S. Belia S. Vecchiet J, Morabilo C, Veechiet Land Fano C; Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscul Disord 13: 479-484. 2003.
19 Radak Z, Puesok S. Mcseki S. Csoni T and Ferdinandy P; Muscle soreness-induced reduclion in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med 26: 1059-1063. 1996.
20 Ber,,stein M, Morabia A and Sloutskis 0: Deflnition and prevalence of sedentarism in an urban population. Am .1 Public Health 89: 862-867, 1999.