uglevod
Senior Member
- Messages
- 220
Interesting research explaining why some CFS(and other chronic diseases) sufferers have their symptoms changed(to worse or better) with Lactoferrin.
In a few words: Lactoferrin is capable of upregulating suppressed immune system in the context of infection/toxins. Below it was used in the model of fungal infection(candida), but innate immunity reacts towards the whole set of acute(extracellular) pathogens more or less the same way(stimulation of TLR receptors -> pro inflammatory response -> pathogens clearance with phagocytosis):
Effect of orally administered bovine lactoferrin on the immune response in the oral candidiasis murine model
https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.05505-0
Therapeutic activity against oral candidiasis of orally administered bovine lactoferrin (LF), a multifunctional milk protein, was shown in a previous report using an immunosuppressed murine model. In the present study, the influence of orally administered LF on immune responses relevant to this therapeutic effect was examined. Because mice were immunosuppressed with prednisolone 1 day before and 3 days after the infection with Candida, the numbers of peripheral blood leukocytes (PBL) and cervical lymph node (CLN) cells were reduced. Lactoferrin feeding prevented the reduction in the numbers of peripheral blood leukocytes on day 1 and CLN cells on days 1, 5 and 6 in the Candida-infected mice. The number of CLN cells of individual mice on days 5 and 6 was inversely correlated with the Candida c.f.u. in the oral cavity. Increased production of IFN-γ and TNF-α by CLN cells stimulated with heat-killed Candida albicans on day 6 was observed in LF-treated mice compared with non-treated mice. Concanavalin A (ConA)-stimulated CLN cells from LF-treated mice also showed a significant increase in the production of IFN-γ and IL12 on day 5 and a tendency for increased production of IFN-γ and TNF-α on day 6. The levels of cytokine production by ConA-stimulated CLN cells on day 6 were inversely correlated with the Candida c.f.u. in the oral cavity. In conclusion, the alleviation of oral candidiasis by LF feeding in this model may correlate with the enhancement of the number of leukocytes and their cytokine responses in regional lymph nodes against Candida infection.
Oral candidiasis, caused by C. albicans, is most prevalent in infancy and old age and in individuals with immunosuppressive conditions. The clinical importance of oral candidiasis, which is not life-threatening but causes significant morbidity in patients, has increased recently (Hermann et al., 2001). Some drugs such as azole antifungal agents are used for chemotherapy of this fungal infection (Walsh et al., 2000), but long-term treatments sometimes lead to the appearance of drug-resistant Candida and side effects (Lopez-Ribot et al., 1999). Recently, we developed a new oral candidiasis model using immunosuppressed mice; the mice in this model have local symptoms characteristic of oral thrush (Takakura et al., 2003a). In an assessment of the potential of LF as a food component using this animal model, it was demonstrated that LF feeding improved oral candidiasis microbiologically and symptomatically (Takakura et al., 2003b). In that study, it was suggested that the effect of LF in this oral candidiasis model is not attributable simply to its direct antifungal activity. Therefore, the influence of orally administered LF on systemic or local immune responses relevant to its therapeutic effect in this model was examined in the present study.
On the other hand, there was no difference in the number of neutrophils in PBL between the two groups. This increase of lymphocytes in PBL by LF is in accord with the previous finding by Kuhara et al. (2000), that orally administered LF increased the number of cells in various lymphocyte subsets, including CD4+, CD8+ and asialoGM1+ cells, in the peripheral blood of tumour-bearing mice. CLNs are the most important lymph nodes in protection against oral infection (Elahi et al., 2000). The reduction in the number of CLN cells on day 1 was prevented by orally administered LF, as seen in PBL (Fig. 1d).
The enhancement by LF treatment of the number of PBL and CLN cells on day 1 was observed only in mice infected with Candida and also treated with PDS, and not in mice infected only, in mice treated with PDS only or in normal mice (Fig. 2a, b). Artym et al. (2003) demonstrated that the drop in the number of PBL and the strong reduction in the percentage of lymphocytes induced by cyclophosphamide, which is known to be a potent immunosuppressive drug, were prevented by orally administered LF in mice. In their study, LF decreased the number of PBL when administered to normal mice. Therefore, we can assume that oral LF may affect lymphocyte numbers in PBL and CLN cells in a manner dependent on the host's immune status.
Cytokine production by CLN cells stimulated with ConA or heat-killed C. albicans
After CLN cells were collected on day 5 or 6 and stimulated with ConA or heat-killed C. albicans, the levels of cytokines in the culture supernatants were measured. In this study, CLN cells were cultured for 4 days, because cytokine production was reduced by PDS treatment. On day 5, the level of IFN-γ and IL12 production by CLN cells stimulated with ConA was higher in LF-treated mice than in non-treated mice (Fig. 4). The level of production of IFN-γ by CLN cells stimulated with ConA was notably increased on day 6 compared with day 5, whereas that of IL12 was decreased. Augmentation of IFN-γ but not IL12 production by LF on day 6 was observed in CLN cells stimulated with Candida cells. Enhancement of TNF-α production by LF administration was also clearly seen on day 6 in CLN cells stimulated with Candida. IL2 production seemed somewhat higher in the LF group, but the difference between the two groups was not significant. Moreover, comparison of cytokine production by CLN cells and Candida c.f.u. in the oral cavity of individual mice on day 6 indicated that the levels of IFN-γ and TNF-α production after stimulation with ConA were inversely correlated with Candida c.f.u. (IFN-γ: r = −0.75, P = 0.02; TNF-α: r = −0.83, P = 0.01) and IL12 production by these cells was correlated with the number of C. albicans cells in the oral cavity (r = 0.70, P = 0.06).
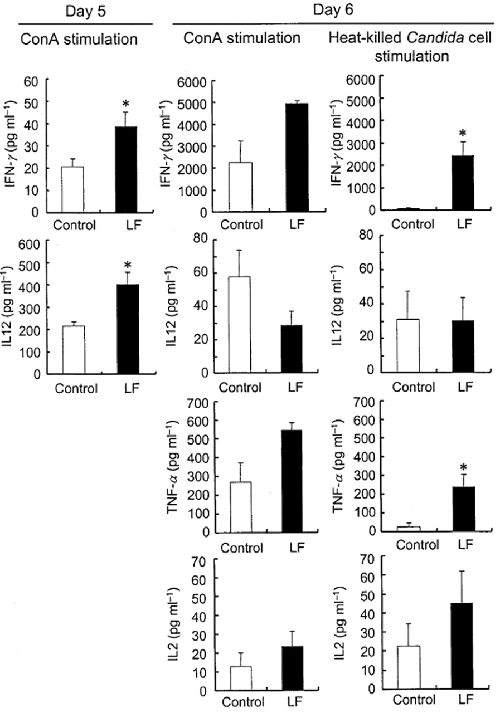
It is well known that TNF-α and IFN-γ stimulate macrophages and neutrophils, which kill C. albicans directly (Tansho et al., 1994). Farah et al. (2002) demonstrated that TNF-α in oral tissue was an important mediator in the recovery from oropharyngeal candidiasis in mice. Their findings suggest that T lymphocyte infiltration into the oral tissue activates resident or accumulated macrophages and neutrophils by releasing TNF-α and inducing a phagocytic response against invading hyphal elements. CLN cells activated by LF feeding may migrate to the circulation, with some reaching the oral mucosa. Further examination is necessary of the influence of LF feeding on immunological events in the oral tissue using this model. In the past few years, several immunomodulatory effects of ingested LF in animals have been reported (Sekine et al., 1997; Wakabayashi et al., 2002). Very recently, we showed that LF feeding augmented macrophage activity at the local site where inactivated Candida was injected as a priming agent (Wakabayashi et al., 2003). These results support the possibility that LF feeding upregulates immune functions of effector cells and causes the early resolution of oral candidiasis in this model.
As shown by clinical observations and experimental data in animal models, impairment of cell-mediated immunity can enhance susceptibility to mucosal candidal infections (Fidel, 2002). Therefore, upregulation of cell-mediated immunity by LF feeding, as indicated by the enhancement of Th1-type cytokine production, may be beneficial for the treatment of oral candidiasis. Masci (2000) has already reported that a mouthwash containing LF and lysozyme was effective against oral candidiasis in an HIV patient. Our findings suggest that LF from cows’ milk can be used not only as a mouthwash but also as a dietary supplement with immunomodulatory action for antifungal treatment.
In a few words: Lactoferrin is capable of upregulating suppressed immune system in the context of infection/toxins. Below it was used in the model of fungal infection(candida), but innate immunity reacts towards the whole set of acute(extracellular) pathogens more or less the same way(stimulation of TLR receptors -> pro inflammatory response -> pathogens clearance with phagocytosis):
Effect of orally administered bovine lactoferrin on the immune response in the oral candidiasis murine model
https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.05505-0
Therapeutic activity against oral candidiasis of orally administered bovine lactoferrin (LF), a multifunctional milk protein, was shown in a previous report using an immunosuppressed murine model. In the present study, the influence of orally administered LF on immune responses relevant to this therapeutic effect was examined. Because mice were immunosuppressed with prednisolone 1 day before and 3 days after the infection with Candida, the numbers of peripheral blood leukocytes (PBL) and cervical lymph node (CLN) cells were reduced. Lactoferrin feeding prevented the reduction in the numbers of peripheral blood leukocytes on day 1 and CLN cells on days 1, 5 and 6 in the Candida-infected mice. The number of CLN cells of individual mice on days 5 and 6 was inversely correlated with the Candida c.f.u. in the oral cavity. Increased production of IFN-γ and TNF-α by CLN cells stimulated with heat-killed Candida albicans on day 6 was observed in LF-treated mice compared with non-treated mice. Concanavalin A (ConA)-stimulated CLN cells from LF-treated mice also showed a significant increase in the production of IFN-γ and IL12 on day 5 and a tendency for increased production of IFN-γ and TNF-α on day 6. The levels of cytokine production by ConA-stimulated CLN cells on day 6 were inversely correlated with the Candida c.f.u. in the oral cavity. In conclusion, the alleviation of oral candidiasis by LF feeding in this model may correlate with the enhancement of the number of leukocytes and their cytokine responses in regional lymph nodes against Candida infection.
Oral candidiasis, caused by C. albicans, is most prevalent in infancy and old age and in individuals with immunosuppressive conditions. The clinical importance of oral candidiasis, which is not life-threatening but causes significant morbidity in patients, has increased recently (Hermann et al., 2001). Some drugs such as azole antifungal agents are used for chemotherapy of this fungal infection (Walsh et al., 2000), but long-term treatments sometimes lead to the appearance of drug-resistant Candida and side effects (Lopez-Ribot et al., 1999). Recently, we developed a new oral candidiasis model using immunosuppressed mice; the mice in this model have local symptoms characteristic of oral thrush (Takakura et al., 2003a). In an assessment of the potential of LF as a food component using this animal model, it was demonstrated that LF feeding improved oral candidiasis microbiologically and symptomatically (Takakura et al., 2003b). In that study, it was suggested that the effect of LF in this oral candidiasis model is not attributable simply to its direct antifungal activity. Therefore, the influence of orally administered LF on systemic or local immune responses relevant to its therapeutic effect in this model was examined in the present study.
On the other hand, there was no difference in the number of neutrophils in PBL between the two groups. This increase of lymphocytes in PBL by LF is in accord with the previous finding by Kuhara et al. (2000), that orally administered LF increased the number of cells in various lymphocyte subsets, including CD4+, CD8+ and asialoGM1+ cells, in the peripheral blood of tumour-bearing mice. CLNs are the most important lymph nodes in protection against oral infection (Elahi et al., 2000). The reduction in the number of CLN cells on day 1 was prevented by orally administered LF, as seen in PBL (Fig. 1d).
The enhancement by LF treatment of the number of PBL and CLN cells on day 1 was observed only in mice infected with Candida and also treated with PDS, and not in mice infected only, in mice treated with PDS only or in normal mice (Fig. 2a, b). Artym et al. (2003) demonstrated that the drop in the number of PBL and the strong reduction in the percentage of lymphocytes induced by cyclophosphamide, which is known to be a potent immunosuppressive drug, were prevented by orally administered LF in mice. In their study, LF decreased the number of PBL when administered to normal mice. Therefore, we can assume that oral LF may affect lymphocyte numbers in PBL and CLN cells in a manner dependent on the host's immune status.
Cytokine production by CLN cells stimulated with ConA or heat-killed C. albicans
After CLN cells were collected on day 5 or 6 and stimulated with ConA or heat-killed C. albicans, the levels of cytokines in the culture supernatants were measured. In this study, CLN cells were cultured for 4 days, because cytokine production was reduced by PDS treatment. On day 5, the level of IFN-γ and IL12 production by CLN cells stimulated with ConA was higher in LF-treated mice than in non-treated mice (Fig. 4). The level of production of IFN-γ by CLN cells stimulated with ConA was notably increased on day 6 compared with day 5, whereas that of IL12 was decreased. Augmentation of IFN-γ but not IL12 production by LF on day 6 was observed in CLN cells stimulated with Candida cells. Enhancement of TNF-α production by LF administration was also clearly seen on day 6 in CLN cells stimulated with Candida. IL2 production seemed somewhat higher in the LF group, but the difference between the two groups was not significant. Moreover, comparison of cytokine production by CLN cells and Candida c.f.u. in the oral cavity of individual mice on day 6 indicated that the levels of IFN-γ and TNF-α production after stimulation with ConA were inversely correlated with Candida c.f.u. (IFN-γ: r = −0.75, P = 0.02; TNF-α: r = −0.83, P = 0.01) and IL12 production by these cells was correlated with the number of C. albicans cells in the oral cavity (r = 0.70, P = 0.06).
It is well known that TNF-α and IFN-γ stimulate macrophages and neutrophils, which kill C. albicans directly (Tansho et al., 1994). Farah et al. (2002) demonstrated that TNF-α in oral tissue was an important mediator in the recovery from oropharyngeal candidiasis in mice. Their findings suggest that T lymphocyte infiltration into the oral tissue activates resident or accumulated macrophages and neutrophils by releasing TNF-α and inducing a phagocytic response against invading hyphal elements. CLN cells activated by LF feeding may migrate to the circulation, with some reaching the oral mucosa. Further examination is necessary of the influence of LF feeding on immunological events in the oral tissue using this model. In the past few years, several immunomodulatory effects of ingested LF in animals have been reported (Sekine et al., 1997; Wakabayashi et al., 2002). Very recently, we showed that LF feeding augmented macrophage activity at the local site where inactivated Candida was injected as a priming agent (Wakabayashi et al., 2003). These results support the possibility that LF feeding upregulates immune functions of effector cells and causes the early resolution of oral candidiasis in this model.
As shown by clinical observations and experimental data in animal models, impairment of cell-mediated immunity can enhance susceptibility to mucosal candidal infections (Fidel, 2002). Therefore, upregulation of cell-mediated immunity by LF feeding, as indicated by the enhancement of Th1-type cytokine production, may be beneficial for the treatment of oral candidiasis. Masci (2000) has already reported that a mouthwash containing LF and lysozyme was effective against oral candidiasis in an HIV patient. Our findings suggest that LF from cows’ milk can be used not only as a mouthwash but also as a dietary supplement with immunomodulatory action for antifungal treatment.
