godlovesatrier
Senior Member
- Messages
- 2,628
- Location
- United Kingdom
This article on EonNutrition discusses the use of High Dose Thiamine on enzyme processes in the body (Recently mentioned In Cort's HR article. Even if a deficiency isn't present high dose Thiamine appears to exert influence over several enzyme processes. It also discusses KGDH which was mentioned in the study posted on PR here last week.
Date: Nov 2020
https://www.eonutrition.co.uk/post/mega-dose-thiamine-beyond-addressing-deficiency
Author: Elliot Overton
The below quotes are abstracted from the main article:


Date: Nov 2020
https://www.eonutrition.co.uk/post/mega-dose-thiamine-beyond-addressing-deficiency
Author: Elliot Overton
The below quotes are abstracted from the main article:
Overview: " I have since come to the conclusion that one does not need to be deficient (in the nutritional sense) to benefit from this type of therapy, because high-dose thiamine is not simply working by correcting nutritional deficiency. Rather, thiamine is functioning as a metabolic stimulant to restore oxidative energy metabolism in cells that has been inhibited by factors unrelated to nutritional status. "
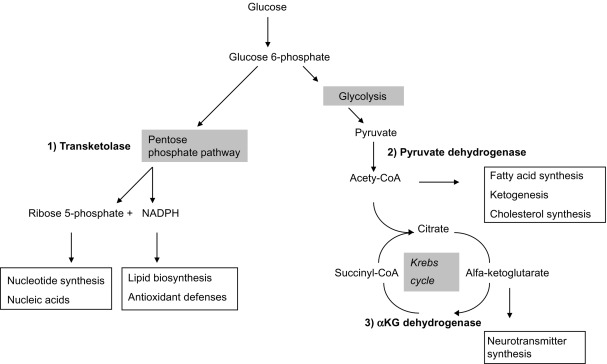
A key enzyme involved in mitochondrial energy metabolism called alpha ketoglutarate dehydrogenase (KGDH). Several nutrients serve as cofactors for this enzyme complex, with thiamine taking center stage. KGDH is a rate-limiting step in in the TCA cycle, meaning that when this enzyme slows down, every other downstream step also slows down. Whilst a deficiency of any of the necessary cofactors will reduce the activity of this enzyme, it is also exquisitely sensitive to oxidative stress.
By saturating the cell, you can bypass the low affinity and restore enzyme function back to its normal state. Extremely high doses are often required to achieve this effect and this therapy must be maintained lifelong.
Examples of nutrient-responsive genetic conditions include:
- Thiamine-responsive maple syrup urine disease: A genetic defect in the branched chain ketoacid dehydrogenase enzyme results in remarkably low affinity for its coenzyme TPP. Continued high doses are necessary restore the function of this enzyme complex.
- Thiamine responsive Leigh’s disease: Inherited mutation in the gene encoding Pyruvate Dehydrogenase, with a decreased affinity for its TPP cofactor. Treated with pharmacological doses of thiamine to stimulate defective enzyme activity
- B12-responsive Methylmalonic acidaemia: Genetic defect encoding the methylmalonyl-CoA mutase enzyme, causing low affinity for adenosylcobalamin cofactor and a pathological accumulation of methylmalonic acid. This condition can be treated with megadoses of B12.
- Biotin-responsive holocarboxylase synthetase deficiency: Genetic mutation renders biotin-responsive carboxylase enzymes much less able to bind with biotin cofactor due to markedly decreased affinity. Supraphysiologic doses can restore normal enzyme function
- B6-responsive homocysteinuria: A rare defect in the cystathionine-B-synthase enzyme reduces affinity for its coenzyme pyridoxal-5-phosphate. This leads to the toxic buildup of homocysteine. Mega-doses of vitamin B6 can return enzyme activity back to normal.
It is worth noting at this point that these genuine genetic defects are extremely rare and are not applicable to the large majority of people. However, similar principles can also be applied when an enzyme has been inactivated by other factors.
However, administering massive doses of thiamine to the rats before TBI was able to completely protect the KGDH enzyme. The thiamine-treated group maintaining normal activity of KGDH, mitochondrial respiration, and ATP despite being exposed to the injury.
Furthermore, the restoration/protection of KGDH might have also conferred some degree of cytoprotection by combating inflammation, which was demonstrated by reduced inflammatory gene expression at 3 days post-TBI.
Human evidence
The late Italian neurological A. Constantini published several case studies on the use of mega doses of thiamine for different conditions and saw impressive results.
In one of the case reports on fibromyalgia, two patients saw an abrupt and immediate improvement only when they reached 1,800mg per day. At lower doses, improvements were negligible.
Constantini hit the nail on the head with one quote from another paper:
“ We may suppose that symptoms decrease when the energetic metabolism and other thiamine-dependent processes return to physiologic levels. Our aim was not to correct a systemic deficit of thiamine, but rather to increase the activity of enzymes involved in cell production energy in selective brain regions;
Thiamine and benfotiamine supplements
exhibit “anti-stress” properties in the brain, protecting against stress induced suppression of hippocampal neurogenesis. These effects stem from anti-oxidant, rather than coenzyme roles.
Here are a list of some of the non-coenzyme targets of high dose Benfotiamine: