4/9/2020 - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144665/#!po=17.3077 - “Ongoing Clinical Trials for the Management of the COVID-19 Pandemic”.
Note - This study review has also been posted under a thread re anti-virals, see - https://forums.phoenixrising.me/thr...gainst-coronavirus.79014/page-14#post-2270018 . Reposting it here because it identifies over 100 other treatments besides the studies testing antivirals. A number of other treatment possibilities have threads/posts here on the Forum such as hydroxychloriquine, Vitamin C, Nitric Oxide, Stem Cells, Hydrogen Peroxide, Convalescence Serum etc... I thought people might be interested in seeing all these different types of treatment ideas that are currently part of a clinical trial.
Note - This study review has also been posted under a thread re anti-virals, see - https://forums.phoenixrising.me/thr...gainst-coronavirus.79014/page-14#post-2270018 . Reposting it here because it identifies over 100 other treatments besides the studies testing antivirals. A number of other treatment possibilities have threads/posts here on the Forum such as hydroxychloriquine, Vitamin C, Nitric Oxide, Stem Cells, Hydrogen Peroxide, Convalescence Serum etc... I thought people might be interested in seeing all these different types of treatment ideas that are currently part of a clinical trial.
Currently, there are no approved therapies for either the treatment or prevention of COVID-19. With the predicted number of cases set to rise significantly, this represents a prodigious acute unmet medical need. Several national and international research groups are working collaboratively on a variety of preventative and therapeutic interventions. Potential avenues being explored include vaccine development, convalescent plasma, interferon-based therapies, small-molecule drugs, cell-based therapies, and monoclonal antibodies (mAbs) [5]. However, drug therapy development is a costly and timely process with a high attrition rate [6]. The speed of the normal drug development pathway is unacceptable in the context of the current global emergency. Therefore, there has been considerable interest in repurposing existing drugs and expediting developmental antiviral treatments, such as those for influenza, hepatitis B (HBV), hepatitis C (HCV), and filoviruses, to allow more rapid development [5]. The swift genomic sequencing of COVID-19 has facilitated this process, allowing comparison with MERS-CoV, SARS-CoV, and other morbific viruses [7]. This strategy has identified several genomic regions of interest for therapeutic modulation, specifically the identification of highly conserved regions involving viral enzymes between different pathogenic coronaviruses.
Exploring Current Clinical Trials for Covid-19
Since 2005, it has been recommended by the International Committee of Medical Journal Editors (ICMJE) that all clinical trials should be registered in publicly available domains before they may be considered for publication [8]. The introduction of this requirement and other initiatives to increase clinical trial transparency has contributed to an increasing number of trials being recorded in online registries, such as ClinicalTrials.govv and the International Clinical Trials Registry Platform (ICTRP)vi of the WHO. The logging of trials on registries has vastly facilitated the dissemination of information across several domains, including intervention, methodology, patient group, and outcome measures. Furthermore, in the event of the nonpublication of results, it means that trial information remains freely available for analysis.
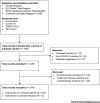
In the context of the current global COVID-19 pandemic, we performed an analysis of online registries (ClinicalTrials.govv, WHO ICTRPvi, EU Clinical Trials Registervii, and Cochrane Central Register of Controlled Trialsviii; Figure 1 ) to collate all registered therapeutic and preventative interventions under clinical investigation. We hope that this will clarify current investigational advances and guide potential future strategies. We identified 344 interventional studies focusing on both preventative strategies and the treatment of patients with COVID-19 (Figure 1) as of 20 March 2020. This search identified 100 studies that focused on forms of traditional Chinese medicine (TCM), including herbal medicines, acupuncture and other forms of complementary medicine. These have not been further analysed due to a lack of scientific rationale, inadequate provision of information regarding active ingredients, and limited applicability to mainstream medical practice. Table 1 (Key Table) shows interventional treatments (Table 1A) and preventative strategies (Table 1B) under clinical investigation for COVID-19.
Figure 1
Flow Diagram Showing the Study Selection Process of Clinical Trials Discussed in This Article and Listed in Table 1 in the Main Text.
Table 1
Key Table. Ongoing Clinical Trials for the (A) Treatment and (B) Prevention of COVID-19 (Current as of 20 March, 2020)a
. . .