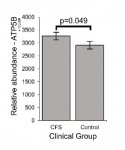
There is a European study which has checked for the expression of 1007 mitochondrial proteins in platelets from 2 twins, one with ME/CFS and the other one healthy (Ciregia F et al 2016). Of these proteins, 194 were significantly modified in the sick twin, in comparison with the healthy one. I have checked for differencies in pyruvate dehydrogenase complex, ADP/ATP translocase subunits and pyruvate dehydrogenase kinases. This is what I have found in these two twins:
1) Pyruvate dehydrogenase E1 subunits alpha (PDHA) and beta are both inceased in the sick twin, which is in partial accordance with the increse in PDHA found by Fluge and Mella (Fluge et al. 2016);
2) ADP/ATP translocase, sub unit 2 and 3, are low in the sick twin, compared with the healthy one, which could be in accordance with the study by Myhill and colleagues (Myhill S et al. 2009), (Booth, N et al 2012), if only we assume that the problem with this enzyme found in group HiBlk is not due to blockage from a molecule or an autoantibody, but is instead due to under expression of the enzyme itself (@Hip);
3) Pyruvate dehydrogenase kinases 1 and 3 are over expressed, which again is in partial accordance with what FLuge and Mella have found in their recent paper, where PDK 1, 2, 4 are over expressed in ME/CFS patients.
In conclusion, the sick twin does not seem to have any blockage of ADP/ATP translocase, because if that was true he would have an over expression of the enzyme, while the enzyme is under expressed. On the other hand he does seem to have a problem with his pyruvate dehydrogenase, in fact there is inhibition by over expressed PDK 1 and 3 and - at the same time - he is expressing more PDHA than his heathy twin.
1) Pyruvate dehydrogenase E1 subunits alpha (PDHA) and beta are both inceased in the sick twin, which is in partial accordance with the increse in PDHA found by Fluge and Mella (Fluge et al. 2016);
2) ADP/ATP translocase, sub unit 2 and 3, are low in the sick twin, compared with the healthy one, which could be in accordance with the study by Myhill and colleagues (Myhill S et al. 2009), (Booth, N et al 2012), if only we assume that the problem with this enzyme found in group HiBlk is not due to blockage from a molecule or an autoantibody, but is instead due to under expression of the enzyme itself (@Hip);
3) Pyruvate dehydrogenase kinases 1 and 3 are over expressed, which again is in partial accordance with what FLuge and Mella have found in their recent paper, where PDK 1, 2, 4 are over expressed in ME/CFS patients.
In conclusion, the sick twin does not seem to have any blockage of ADP/ATP translocase, because if that was true he would have an over expression of the enzyme, while the enzyme is under expressed. On the other hand he does seem to have a problem with his pyruvate dehydrogenase, in fact there is inhibition by over expressed PDK 1 and 3 and - at the same time - he is expressing more PDHA than his heathy twin.
Last edited: