View the Post on the Blog
Action for M.E. - the UK's largest CFS/ME charity - launched a new research strategy in November based on the priorities identified by patients. We asked the Charity's Chief Executive, Sonya Chowdhury, about the new strategy and also about the commitment to greater patient involvement. By Russell Fleming and Simon McGrath.

Sonya Chowdhury, CEO, Action for M.E.Charities have always connected with patients - many were founded by patients and are supported with patient help, but few have tried to give patients a voice when it comes to decision making.
Action for M.E. is working to do just that, a task made far more practical by the internet: consultation has never been so easy, so fast or so cheap.
At its Research Conference held at the beginning of November, Sonya Chowdhury the Chief Executive, announced a new research strategy, and what made it different, unique even, was that its' priorities had all been determined by patients.
The New Research Strategy
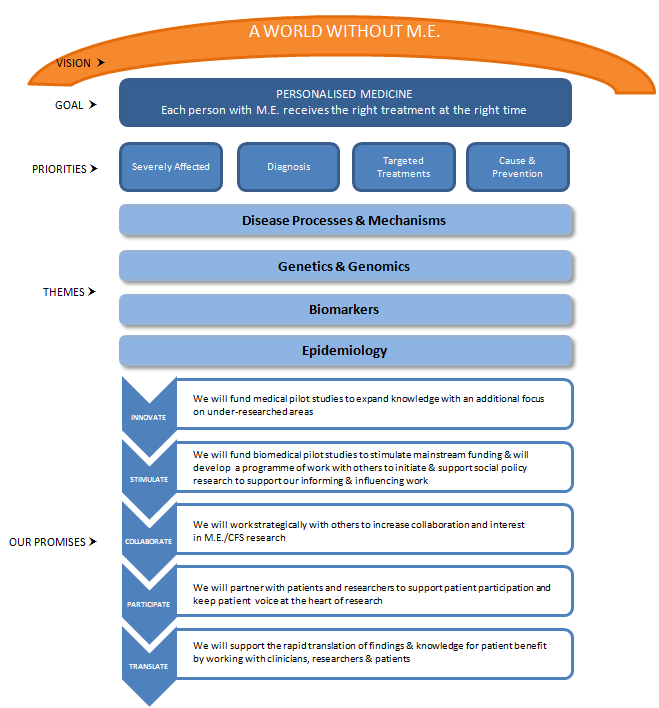
The new strategy is summarised in the graphic below and is perhaps worth taking some time to consider:
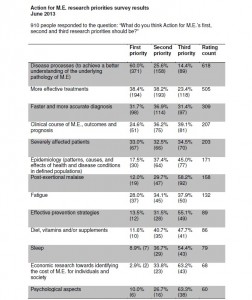
The priorities and themes were drawn from the top 5 patient priorities - determined by consulting the community through a survey in June, which attracted the views of 1,000 people (900 of whom had M.E.). Together, these 5 priorities won 85% of patient first-choice votes:
Two promises worth highlighting are, that:
“We will fund biomedical pilot studies to stimulate mainstream funding and will develop a programme of work with others to initiate and support social policy research to support our informing and influencing work”
and
“We will partner with patients and researchers to support patient participation and keep patient voice and the heart of research”
Committed to consultation
The patient survey served to also inform the Executive Board of the UK CFS/ME Research Collaborative which had initially sought patient feedback. However, this canvassing of opinion, is not to be seen as a one-off exercise, as Sonya was quick to explain:
“We will do the same again when we set any new strategy, but that’s not to say that we wouldn’t do something sooner if there was need or something changes significantly. There has to be a level of flexibility and agility in all of our strategies and policies.
We will, however, continue to pursue our commitment to patient engagement, to participation and to giving patient's a voice, and we will ensure that we listen on many different levels, for example through discussion threads, questions on Facebook and more surveys.
We have a list of ideas from various discussions we've already had such as establishing a patient reference group, focus groups (actual and virtual), and more regular consultations.”
We asked Sonya some more about the new strategy, the charity's approach to research and what consulting patients might actually mean in practice:
What do you see as the most important thing(s) in the new research strategy? And why?
“I strongly believe in the need for personalised medicine and anything that helps us get to that point has to be of importance. If I had to pick two promises, or core values that underpin the strategy, these would be collaboration and participation.
I have a background in children’s rights and ensuring meaningful engagement/participation and I fundamentally believe that you achieve the best possible outcomes by working with the people that you work on behalf of.
This isn’t an optional extra; meaningful engagement and consultation with our supporting members and others, such as other M.E. charities and patient groups, has to be inherent in our work.
I also believe that we have to work collaboratively if we are to achieve the level of transformation needed for people affected by ME/CFS. That includes people with whom we may have differing views as long as we can find some common ground that is in service of achieving positive change.”
Several of the patient-determined priorities have been talked about for a long time now, for example the need to focus on severe patients, epidemiology, and biomarkers. Why do you think it has it taken so long to act upon them? And how do you think highlighting them again in this way will make a difference?
“Action for M.E. has funded work in these areas and the UK ME/CFS Biobank that we’re co-funding is collating samples including from people who are more severely affected. However, there is still a significant gap and we must increase our focus on this.
It is not right that people with M.E. are so severely disadvantaged and that there is an imbalance in mainstream funding for research. It’s even worse that we have those who are more severely affected receiving less of a focus with limited or no services and a dearth of research.
You don’t really find that with other illnesses. A researcher once described people in this group as ‘severely affected, severely neglected’. It’s unjust and therefore we have to continue not only highlighting them, but work with others to turn this into action by securing more money and more research.”
Does this strategy represent a change in direction for Action for M.E. in terms of research funding and if so, in what way? For example, your website mentions 'biomedical', is this now the sole determinant for qualification, to the exclusion of, say, psychological research which came last in your survey as a first choice for patients?
“Our recent funding has been used for biomedical research and so this isn’t a change of direction. What we have sought to do is to be explicit about what we do and how we do it.
There is such a momentum at present within the research field and therefore it is right to review our position and work in this area. We are also continuing our commitment to being more transparent about our work.
Our assessment for which research projects we will fund, partner and/or support is focused on, “how might this benefit people with M.E.?” and you can read our assessment criteria for partnering requests.
We should never rule anything out if there is a strong case for supporting a project, but our focus currently is on biomedical research projects.”
How will you be actively seeking research projects and promoting CFS/ME as an exciting area of interest?
“We have circulated our 'Call for Proposals' as widely as possible across our network of researchers and universities as well as through social media and our Online M.E. Centre.
I have already had discussions with a number of researchers interested in submitting applications including some who are at present outside the CFS/ME field.
There are many things that make CFS/ME an exciting area of interest. Not only do you have the technological and social advances that are benefiting research more widely, but there is increasing collaboration in this field with many new discoveries yet to be made, and there is the opportunity to make a significant difference.
True, the same can be said for a number of other illness areas. However, when I talk to researchers who have recently started working in the CFS/ME field, the thing that many say is different is the higher level of patient engagement, and the commitment and passion to work in a meaningful way with researchers.”
Why do you think there remains a need for small, and potentially fragmented 'pilot' studies when the field is dominated by such research dating back 50 years? Isn't it time we looked to more significant, larger, and better defined research, that includes perhaps the replication of existing research?
“We would love to be in a position to fund larger projects but we’re not. We are not just a research charity and also need funds to deliver services which provide information and support to more than 300,000 people.
We also believe that CFS/ME research is a priority, given the injustice, ignorance and neglect that exists and therefore larger research projects should have mainstream funding.
Our role is to add to, or complement mainstream funding through investing in feasibility studies and so forth. It is essential, though, that there is better collaboration between researchers so that the potential benefit from pilot projects is realised.”
“Stephen has been a member of our Research Panel since it was launched in January 2012. He plays a significant role in the Panel and has contributed to all of our meetings and discussions.
The Panel, which also features three supporting members chosen by their peers, reports to the Board and oversees our research work as well as helping set the strategy, identify which projects to fund and/or partner and helps to ensure accountability for the funding that we invest.
Personally, I find Stephen incredibly supportive as a sounding-board and in offering guidance. We have a strong Research Panel and Stephen is very much a part of this.”
Involving patients appears then to be a continuing focus for your work, so what's the next step for the strategy and for continuing patient participation?
“We want to discover what our supporting members and others think about where we’ve got to and develop our plan to put this strategy into action.
We are also keen to hear how we can further engage with our supporting members and people affected by M.E. to inform our research work.
So, if anyone reading this has any views, thoughts, ideas or questions, please do contact Action for M.E., or you contact me direct.
We can always do better but I hope we are making good progress on really listening, responding and acting on what our supporting members and others affected by M.E. have to say.”
Phoenix Rising is a registered 501 c.(3) non profit. We support ME/CFS and NEID patients through rigorous reporting, reliable information, effective advocacy and the provision of online services which empower patients and help them to cope with their isolation.
There are many ways you can help Phoenix Rising to continue its work. If you feel able to offer your time and talent, we could really use some more authors, proof-readers, fundraisers, technicians etc. and we'd love to expand our Board of Directors. So, if you think you can help then please contact Mark through the Forum.
And don't forget: you can always support our efforts at no cost to yourself as you shop online! To find out more, visit Phoenix Rising’s Donate page by clicking the button below.
View the Post on the Blog
Action for M.E. - the UK's largest CFS/ME charity - launched a new research strategy in November based on the priorities identified by patients. We asked the Charity's Chief Executive, Sonya Chowdhury, about the new strategy and also about the commitment to greater patient involvement. By Russell Fleming and Simon McGrath.

Sonya Chowdhury, CEO, Action for M.E.
Action for M.E. is working to do just that, a task made far more practical by the internet: consultation has never been so easy, so fast or so cheap.
At its Research Conference held at the beginning of November, Sonya Chowdhury the Chief Executive, announced a new research strategy, and what made it different, unique even, was that its' priorities had all been determined by patients.
The New Research Strategy
The new strategy is summarised in the graphic below and is perhaps worth taking some time to consider:
The priorities and themes were drawn from the top 5 patient priorities - determined by consulting the community through a survey in June, which attracted the views of 1,000 people (900 of whom had M.E.). Together, these 5 priorities won 85% of patient first-choice votes:
- Disease Processes and causes (underlying pathology)
- Better Treatments
- Better Diagnosis
- Clinical course of ME, outcomes and prognosis (Epidemiology)
- Severely Affected patients
Two promises worth highlighting are, that:
“We will fund biomedical pilot studies to stimulate mainstream funding and will develop a programme of work with others to initiate and support social policy research to support our informing and influencing work”
and
“We will partner with patients and researchers to support patient participation and keep patient voice and the heart of research”
Committed to consultation
The patient survey served to also inform the Executive Board of the UK CFS/ME Research Collaborative which had initially sought patient feedback. However, this canvassing of opinion, is not to be seen as a one-off exercise, as Sonya was quick to explain:
“We will do the same again when we set any new strategy, but that’s not to say that we wouldn’t do something sooner if there was need or something changes significantly. There has to be a level of flexibility and agility in all of our strategies and policies.
We will, however, continue to pursue our commitment to patient engagement, to participation and to giving patient's a voice, and we will ensure that we listen on many different levels, for example through discussion threads, questions on Facebook and more surveys.
We have a list of ideas from various discussions we've already had such as establishing a patient reference group, focus groups (actual and virtual), and more regular consultations.”
We asked Sonya some more about the new strategy, the charity's approach to research and what consulting patients might actually mean in practice:
What do you see as the most important thing(s) in the new research strategy? And why?
“I strongly believe in the need for personalised medicine and anything that helps us get to that point has to be of importance. If I had to pick two promises, or core values that underpin the strategy, these would be collaboration and participation.
I have a background in children’s rights and ensuring meaningful engagement/participation and I fundamentally believe that you achieve the best possible outcomes by working with the people that you work on behalf of.
This isn’t an optional extra; meaningful engagement and consultation with our supporting members and others, such as other M.E. charities and patient groups, has to be inherent in our work.
I also believe that we have to work collaboratively if we are to achieve the level of transformation needed for people affected by ME/CFS. That includes people with whom we may have differing views as long as we can find some common ground that is in service of achieving positive change.”
Several of the patient-determined priorities have been talked about for a long time now, for example the need to focus on severe patients, epidemiology, and biomarkers. Why do you think it has it taken so long to act upon them? And how do you think highlighting them again in this way will make a difference?
“Action for M.E. has funded work in these areas and the UK ME/CFS Biobank that we’re co-funding is collating samples including from people who are more severely affected. However, there is still a significant gap and we must increase our focus on this.
It is not right that people with M.E. are so severely disadvantaged and that there is an imbalance in mainstream funding for research. It’s even worse that we have those who are more severely affected receiving less of a focus with limited or no services and a dearth of research.
You don’t really find that with other illnesses. A researcher once described people in this group as ‘severely affected, severely neglected’. It’s unjust and therefore we have to continue not only highlighting them, but work with others to turn this into action by securing more money and more research.”
Does this strategy represent a change in direction for Action for M.E. in terms of research funding and if so, in what way? For example, your website mentions 'biomedical', is this now the sole determinant for qualification, to the exclusion of, say, psychological research which came last in your survey as a first choice for patients?
“Our recent funding has been used for biomedical research and so this isn’t a change of direction. What we have sought to do is to be explicit about what we do and how we do it.
There is such a momentum at present within the research field and therefore it is right to review our position and work in this area. We are also continuing our commitment to being more transparent about our work.
Our assessment for which research projects we will fund, partner and/or support is focused on, “how might this benefit people with M.E.?” and you can read our assessment criteria for partnering requests.
We should never rule anything out if there is a strong case for supporting a project, but our focus currently is on biomedical research projects.”
Action for M.E. is currently funding the following CFS/ME research projects:
- Epidemiology: The Disease Register
- Co-funding the UK's first Biobank
- A study into muscle dysfunction at Newcastle University
- Funding a case-controlled study exploring the qualitative experience of sleep at Northumbria University
- A study looking at cognitive impairment associated with fatigue at the University of Sheffield.
Action for M.E. 2013 Call for Research Proposals
Research Fortnight
Advertisement Nov. 28, 2013
Research Fortnight
Advertisement Nov. 28, 2013
“Action for M.E. offers research grants
Action for M.E. is calling for applications for its pilot research grants; £60,000 is available for up to three projects. Applications should focus on underlying chronic changes related to M.E./CFS, particularly focusing on post-exertional fatigue, autonomic dysfunction, immune dysregulation, phenotyping, epidemiology and severe M.E.”
Research Fortnight, p 10.
Action for M.E. is calling for applications for its pilot research grants; £60,000 is available for up to three projects. Applications should focus on underlying chronic changes related to M.E./CFS, particularly focusing on post-exertional fatigue, autonomic dysfunction, immune dysregulation, phenotyping, epidemiology and severe M.E.”
Research Fortnight, p 10.
“We have circulated our 'Call for Proposals' as widely as possible across our network of researchers and universities as well as through social media and our Online M.E. Centre.
I have already had discussions with a number of researchers interested in submitting applications including some who are at present outside the CFS/ME field.
There are many things that make CFS/ME an exciting area of interest. Not only do you have the technological and social advances that are benefiting research more widely, but there is increasing collaboration in this field with many new discoveries yet to be made, and there is the opportunity to make a significant difference.
True, the same can be said for a number of other illness areas. However, when I talk to researchers who have recently started working in the CFS/ME field, the thing that many say is different is the higher level of patient engagement, and the commitment and passion to work in a meaningful way with researchers.”
Why do you think there remains a need for small, and potentially fragmented 'pilot' studies when the field is dominated by such research dating back 50 years? Isn't it time we looked to more significant, larger, and better defined research, that includes perhaps the replication of existing research?
“We would love to be in a position to fund larger projects but we’re not. We are not just a research charity and also need funds to deliver services which provide information and support to more than 300,000 people.
We also believe that CFS/ME research is a priority, given the injustice, ignorance and neglect that exists and therefore larger research projects should have mainstream funding.
Our role is to add to, or complement mainstream funding through investing in feasibility studies and so forth. It is essential, though, that there is better collaboration between researchers so that the potential benefit from pilot projects is realised.”
Sonya on winning patients' trust
I couldn't help but notice that Professor Stephen Holgate is a member of your Research Panel. Was this a recent appointment and what role does he play?
“It’s now 14 months since I came into post... and what a rollercoaster ride it’s been. At our Research Conference and AGM a couple of weeks ago, I reflected back on my first few months, and commented on how shell-shocked I think I was.
I hadn’t anticipated the level of fragmentation, frustration and anger that existed, much of it understandable given the ignorance, inequalities and actual discrimination that I also saw and heard about.
BUT... I was, and still continue to be, incredibly touched by the passion, the sheer determination and commitment that many people were taking to create the level of change that is so desperately needed.
Over the past year, I have had to front up to criticism and significant challenges to what we have said or done, both past and present. I have also received tremendous praise for things we have done, are doing and plan to do.
It is absolutely right that as a patient charity, we, and me personally, are held to account by our supporting members.
We are working hard to ensure that we engage with people affected by M.E. in a meaningful way to inform our work and to help ensure we continue to do better.
This is set out, in the form of ‘our promises’, in our Statement of Strategic Intent that we launched in May.”“Stephen has been a member of our Research Panel since it was launched in January 2012. He plays a significant role in the Panel and has contributed to all of our meetings and discussions.
The Panel, which also features three supporting members chosen by their peers, reports to the Board and oversees our research work as well as helping set the strategy, identify which projects to fund and/or partner and helps to ensure accountability for the funding that we invest.
Personally, I find Stephen incredibly supportive as a sounding-board and in offering guidance. We have a strong Research Panel and Stephen is very much a part of this.”
Involving patients appears then to be a continuing focus for your work, so what's the next step for the strategy and for continuing patient participation?
“We want to discover what our supporting members and others think about where we’ve got to and develop our plan to put this strategy into action.
We are also keen to hear how we can further engage with our supporting members and people affected by M.E. to inform our research work.
So, if anyone reading this has any views, thoughts, ideas or questions, please do contact Action for M.E., or you contact me direct.
We can always do better but I hope we are making good progress on really listening, responding and acting on what our supporting members and others affected by M.E. have to say.”
Phoenix Rising is a registered 501 c.(3) non profit. We support ME/CFS and NEID patients through rigorous reporting, reliable information, effective advocacy and the provision of online services which empower patients and help them to cope with their isolation.
There are many ways you can help Phoenix Rising to continue its work. If you feel able to offer your time and talent, we could really use some more authors, proof-readers, fundraisers, technicians etc. and we'd love to expand our Board of Directors. So, if you think you can help then please contact Mark through the Forum.
And don't forget: you can always support our efforts at no cost to yourself as you shop online! To find out more, visit Phoenix Rising’s Donate page by clicking the button below.
View the Post on the Blog
Last edited by a moderator: