It is easy to get carried away with confirmation bias as biochemistry is multipurposed and "a theory that explains everything, explains nothing" (the trap of non-specificity).
There is certainly potential for peripheral changes, not merely "fooling the brain", given the links between TGF-Beta and the metabolic findings of Fluge & Mella:
"Reverse crosstalk of TGFβ and PPARβ/δ signaling identified by transcriptional profiling."
https://www.ncbi.nlm.nih.gov/pubmed/20846954
"Transforming growth factor-beta1 is a molecular target for the peroxisome proliferator-activated receptor delta."
https://www.ncbi.nlm.nih.gov/pubmed/18007025
"PPARdelta promotes wound healing by up-regulating TGF-beta1-dependent or -independent expression of extracellular matrix proteins."
https://www.ncbi.nlm.nih.gov/pubmed/19538467
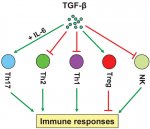
The decreased NK Cell activity findings could be explained by elevated TGF-Beta:
"TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway."
https://www.ncbi.nlm.nih.gov/pubmed/26884601
"Reverse crosstalk of TGFb and PPARb/d signaling identified by transcriptional profiling"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3017614/
"Transforming Growth Factor Beta, Bioenergetics and Mitochondria in Renal Disease"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3444292/
(discusses relationships between energy sensing pathways and TGF-Beta cross regulation)
"TGF-β – an excellent servant but a bad master" (about development of neoplasms)
https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-10-183
"TGF-β: A New Role for an Old AktTOR"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2789270/
"PI3K/mTORC2 regulates TGF-β/Activin signalling by modulating Smad2/3 activity via linker phosphorylation"
https://www.nature.com/articles/ncomms8212
Everyone's favourite hypotheses confirmed.

I don't pretend to understand the feedback loops which would result from all the findings discussed above...