* I gave each line its own paragraphIllness trajectories in the chronic fatigue syndrome: a longitudinal study of improvers versus non-improvers.
J Nerv Ment Dis. 2010 Jul;198(7):486-93.
Ciccone DS, Chandler HK, Natelson BH.
Department of Psychiatry, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, NJ 07103, USA. cicconds@umdnj.edu
Abstract*
The natural progression of chronic fatigue syndrome (CFS) in adults is not well established.
The aims of this longitudinal study were to (a) compare CFS Improvers and Non-Improvers; (b) determine whether an initial diagnosis of fibromyalgia (FM) was associated with CFS nonimprovement; and (c) determine whether this effect could be explained by the presence of nonspecific physical symptoms.
Consecutive referrals to a tertiary clinic that satisfied case criteria for CFS were invited to enroll in a longitudinal study.
After an initial on-site physical examination and psychiatric interview, a total of 94 female care-seekers completed biannual telephone surveys, including the Short Form-36 physical functioning (PF) scale, over a period of 2(1/2) years.
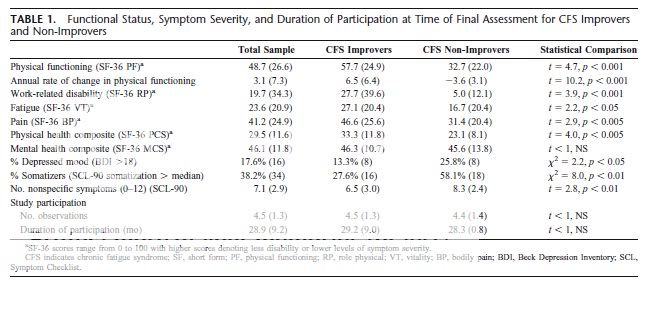
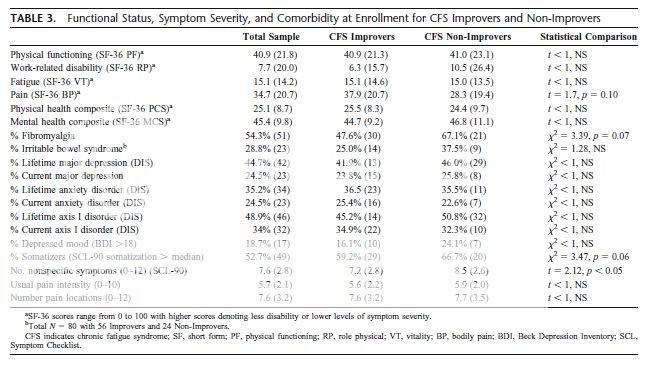
There were very few differences between Improvers and Non-Improvers at baseline but at final assessment Improvers had less disability, less fatigue, lower levels of pain, fewer symptoms of depressed mood, and fewer nonspecific physical complaints.
Participants with FM at baseline were 3.23 times (p < 0.05) more likely to become Non-Improvers than those without FM.
Participants identified initially as Somatizers were 3.33 times (p < 0.05) more likely to become Non-Improvers.
Patients with CFS who bear the added burden of FM are at greater risk of a negative outcome than patients with CFS alone.
This effect could not be explained by the presence of multiple, nonspecific symptoms.
PMID:20611051
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Illness trajectories in the CFS: a longitudinal study of improvers versus non-improve
- Thread starter Dolphin
- Start date
As I put the time into reading it, I thought I'd write a little on it.
Definition of improvement:
----
Definition of somatization/somatizers seems far from exact:
The questions are something like the following (I was looking at a list for the SCL-90-R but I couldn't copy from that so I amalgamated two)
In
Definition of improvement:
It was interesting that the improved group did tend to score higher on quite a few measures even though only this one measure was used.A standardized instrument (the PF subscale of the Short Form SF-36) was administered to study participants at regular 6-month intervals. Improvers were defined as those with a positive illness trajectory in PF (slope>0), whereas Non-Improvers had a negative or flat trajectory (slope <=0) over 2.5 years of study observation.
----
Definition of somatization/somatizers seems far from exact:
Symptom Checklist-90 Somatization Scale
This subscale contains a list of 12 nonspecific medical complaints
often encountered in primary care (Derogatis et al., 1973).
The scale is widely used to assess symptom reporting and has well
established psychometric properties (Derogatis et al., 1976; Lipman
et al., 1979). The patients task is to rate the degree of discomfort
caused by each symptom during the preceding 2 weeks using a
numerical scale ranging from 0 (No Discomfort) to 4 (A Lot of
Discomfort). In the present study, we defined a somatization score
for each participant (at each time point) as the mean rating per item
(sum of symptom ratings divided by number of items rated). We
chose to identify Somatizers on a purely empirical basis as those
with baseline (initial) scores above the median on the Symptom
Checklist-90 Somatization scale.
The questions are something like the following (I was looking at a list for the SCL-90-R but I couldn't copy from that so I amalgamated two)
(copied from: http://www.mindybenowitz.com/images/symptomchecklist.pdf )
Below is a list of problems and experiences that people sometimes have. Read each one carefully and select one of the numbered descriptors that best describes how much that problem has bothered you during the past 2 weeks, including today. Write that number on the line next to the problem. Please do not skip any items, and print your number clearly. Read the example before beginning and if you have any questions, please ask.
EXAMPLE:
How much have you experienced:
1 Not at All
2 A Little Bit
3 Moderately
4 Quite a Bit
5 Extremely
Descriptors:
Headaches.
Feeling dizzy, unsteady, lightheaded or faint.
Pains in heart or chest.
Pains in lower back (doesn't seem to be in another one)
Nausea or upset stomach.
Soreness of your muscles or muscle tension
Trouble getting your breath or smothering sensations.
Chills or hot flushes.
Numbness or tingling in parts of your body.
A lump in your throat.
Feeling weak in parts of your body.
Heavy feeling in your arms or legs.
In
, the authors (i.e. a different team) used this subscale (i.e. the scores from these questions) as a measure of physical symptoms.Priebe S, Fakhoury WK, Henningsen P. In Functional incapacity and physical and psychological symptoms: how they interconnect in chronic fatigue syndrome. Psychopathology. 2008;41(6):339-45. Epub 2008 Sep 3.
Another depressing study set up to confirm the authors own views.
Thanks for digging this out. Could you say what the sample size was, how many improved and how big was the improvement?
As far as I can tell, 4 of these 12 directly relate to the case definition for CFS:
Thanks for digging this out. Could you say what the sample size was, how many improved and how big was the improvement?
As far as I can tell, 4 of these 12 directly relate to the case definition for CFS:
So severity of these might well be expected to correlate with reduced chance of improving.Descriptors:
Headaches.
Soreness of your muscles or muscle tension
Feeling weak in parts of your body.
Heavy feeling in your arms or legs.
Thanks for digging this out. Could you say what the sample size was, how many improved and how big was the improvement?
I find this discussion of outcome measures interesting. It is of course slanted to justify what they did - but whether it's practical or not, I think the bit in bold is interesting:
The also touched on this in the introduction:
A second limitation of the study was
our decision to rely on a single outcome measure (PF) instead of a
retrospective measure of global improvement. As a result, one might
argue that we defined improvement too narrowly. The common
practice of relying on a global rating scale, however, is not without
its own limitations. It presupposes that we (or our patients) know
how to combine information from diverse sources to arrive at an
overall measure of illness outcome. At a minimum, a global measure
should reflect changes in different outcome criteria such as fatigue
severity, pain, physical functioning, medication usage, emotional
distress, employment status, etc. Assuming that patients had accurate
memories for these distant illness-related events, it is still
unlikely that they could integrate them using a reliable or consistent
algorithm, nor is there any reason to believe that the weighting or
scoring rules adopted by one patient would necessarily resemble
those adopted by another. Rather than assume that our patients
perform complex mental arithmetic of this sort, we chose instead to
assess illness outcome along a single behavioral dimension, namely
physical functioning (SF-36 PF). The latter is central to the CFS case
definition which specifies that fatigue alone is not sufficient to
establish a clinical diagnosis (Fukuda et al., 1994). Our emphasis on
physical functioning is also in keeping with the decision by Jason et
al. (2007) to adopt SF-36 PF change scores as their primary outcome
variable in a clinical trial of nonpharmacologic therapy for CFS.
This suggests that clinical improvement is contingent on a change in
physical function and not merely a reduction in fatigue.
Instead of relying on function alone, we could have established
cut-off scores to define a CFS Improver as anyone who
reports a 50% reduction in fatigue coupled with a 50% improvement
in physical function. Such a definition is arbitrary, however, since it
fails to capture those who improve by lesser amounts as well as
those who improve on one dimension but not another. Moreover,
CFS improvement based on 2 or more distinct criteria (such as
physical function and fatigue severity) would not permit an unambiguous
inference about the association between the predictor and a
specific outcome. Had we adopted a multidimensional outcome, for
example, it would not be possible to determine if FM was
associated with improvement in physical function or with a reduction
in fatigue (or with both). In the absence of a consensus, we
chose to define CFS improvement as positive change in a single but
well-defined outcome. As a result, the predictors identified in Table
4 might not apply to outcomes based on other criteria. Since there is
no accepted gold standard for measuring CFS improvement,
efforts to predict outcomes based on alternative criteria would likely
suffer from the same lack of generalizability. Although our definition
of improvement was intentionally narrow, it was nevertheless
associated with changes in multiple illness characteristics, including
pain and fatigue severity, emotional distress, nonspecific symptom
reporting, and work-related impairment (Table 1).
The also touched on this in the introduction:
(Bascially what they seem to be talking about is a regression line - where they may have different numbers of data points depending on how many appointments people went)In virtually all previous longitudinal research, CFS Improvers
have been identified by asking patients at the end of the study
to judge whether they had recovered, remained the same, or gotten
worse. In the present study, we adopted a different approach that did not
depend on the ability of patients to integrate illness-related memories
into overall (global) judgments of CFS gimprovement.h Instead, we
focused on a single dimension of illness outcome, namely perceived
change in physical functioning. A standardized instrument (the PF
subscale of the Short Form SF-36) was administered to study participants
at regular 6-month intervals. Improvers were defined as those
with a positive illness trajectory in PF (slope >0), whereas Non-
Improvers had a negative or flat trajectory (slope <=0) over 21.2 years of
study observation.
They do admit that the model may not be that useful in clinical practice:
I would say they may/will probably need to measure some biological factors.A final limitation is that none of the models presented in
Table 4 provide clinicians with sufficient information to correctly
identify individuals at risk for nonimprovement. This is true despite
the fact that patients with comorbid FM were at least 3 times more
likely to become Non-Improvers. After taking the base rate into
account, as per Altman and Bland (1994), the positive predictive
value for the most complete model (IV) was only 0.536 (with a base
rate of 0.322). On the other hand, the negative predictive value of the
model was 0.806 (with a base rate of 0.677). These rates suggest that
data collected at the time of initial evaluation, including age, current
level of physical functioning, number of nonspecific physical symptoms,
and presence or absence of FM, offer at best only a modest
increment in our ability to detect those at risk. The classification
accuracy of the present model was so poor that only 34% of
Non-Improvers were correctly identified. Effective clinical screening
will require additional research aimed at identifying individual
differences and co-occurring conditions that predict long-term illness
outcomes.
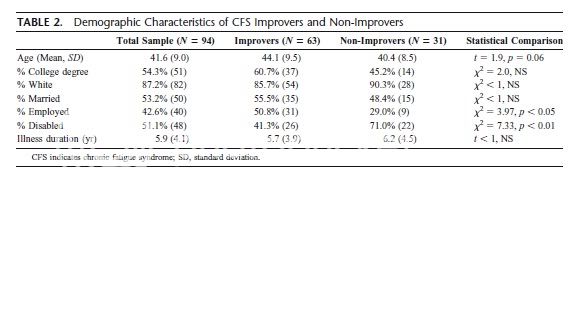
Possible error in Table 2
I just E-mailed this:
I just E-mailed this:
Dear Dr. Ciccone,
I want to check something with you.
I think there is an error in Table 2.
I think the average age for the improvers is probably 40.4 and for non-improvers, 44.1 (it's the opposite in the Table). This would give an mean of 41.6, the mean given
This would chime in more with the findings that:
"Among control variables, increasing age and higher levels of physical functioning at baseline were also associated with nonimprovement."
Bye
Blimey, that's unusually honest of them. And thanks for posting the info I asked for as well as you other comments. It's an interesting-looking study but my brain is a little faded at the moment.They do admit that the model may not be that useful in clinical practice:
I would say they may/will problem need to measure some biological factors.
Esther12
Senior Member
- Messages
- 13,774
Blimey, that's unusually honest of them.
Just from the bits Dolphin pulled out, there were a few parts where they didn't seem to try to cover up the limitations of their work. I like!
re the Somatization scale: My score would really depend upon how much I done in that two week period. If I'd had to push myself, and make myself feel worse, I'd score much higher than if I'd had a couple of weeks of being able to set my own activity levels to an amount I was comfortable with. I'd have thought that many here would be the same.
Yes, and these aren't always under people's own control - different people can have more external pressures of one sort or another (i.e. it's not simply an "internal" matter everyone can always control).re the Somatization scale: My score would really depend upon how much I done in that two week period. If I'd had to push myself, and make myself feel worse, I'd score much higher than if I'd had a couple of weeks of being able to set my own activity levels to an amount I was comfortable with. I'd have thought that many here would be the same.
My guess, based on the way some of the limitations were written it and their placing, is that a reviewer prompted them (they have the feel of stuff that is stuck in a bit). But these aren't the worst team of authors either.
Esther12
Senior Member
- Messages
- 13,774
My guess, based on the way some of the limitations were written it and their placing, is that a reviewer prompted them (they have the feel of stuff that is stuck in a bit). But these aren't the worst team of authors either.
That would be a shame. I'm always excited by hints of intellectual honest in CFS papers. (I can't believe how low my expectations are for CFS research!)
Just been looking at the SCL-90 Somatization subscale myself and wondered if your list was from a source or your assumption having read the list of all 90 Qs?As I put the time into reading it, I thought I'd write a little on it.
Definition of somatization/somatizers seems far from exact:
The questions are something like the following (I was looking at a list for the SCL-90-R but I couldn't copy from that so I amalgamated two):
Descriptors:
Headaches.
Feeling dizzy, unsteady, lightheaded or faint.
Pains in heart or chest.
Pains in lower back (doesn't seem to be in another one)
Nausea or upset stomach.
Soreness of your muscles or muscle tension
Trouble getting your breath or smothering sensations.
Chills or hot flushes.
Numbness or tingling in parts of your body.
A lump in your throat.
Feeling weak in parts of your body.
Heavy feeling in your arms or legs.
In
, the authors (i.e. a different team) used this subscale (i.e. the scores from these questions) as a measure of physical symptoms.
I wasn't sure about the 'lump in throat' question as a somatic item and wondered about these:
Heart pounding or racing
Trembling or shaking
Poor appetite
But it can't be all of them! Any thoughts welcome.
