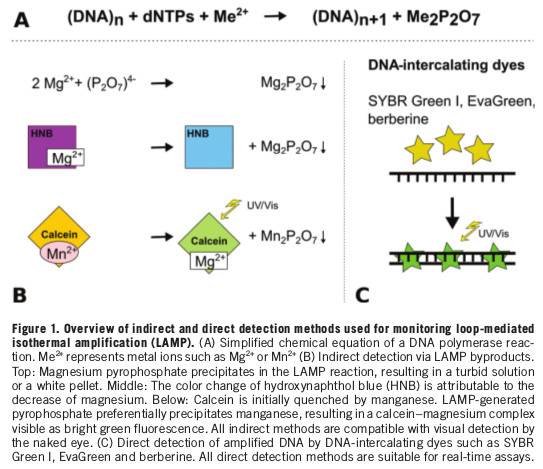
undiagnosed
Senior Member
- Messages
- 246
- Location
- United States
It's clear that the lack access to research assays is primarily political. Ultimately, this problem can be solved by decentralizing the laboratory technologies needed so that people don't need to get permission to perform research. I am starting to seriously investigate the minimum amount of time and money it would require to get a basic setup together to start working on some of my own research questions. The general applications I am interested in, as others here may be, are:
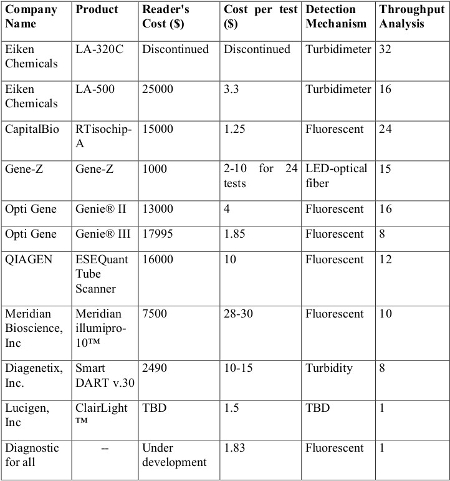
There was an open source project done called the uBAR using LAMP.
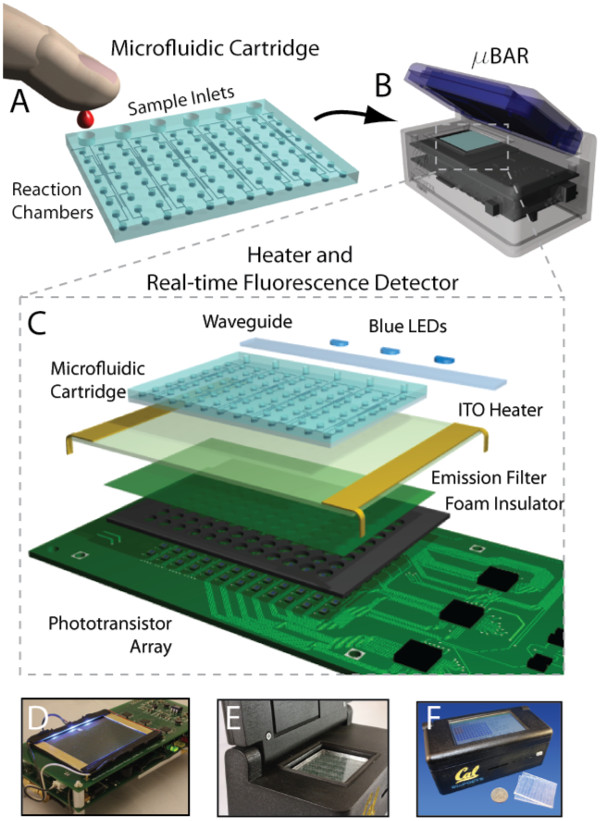
The authors claim prototype units can be built for $919 in single unit quantities. The unit automates analysis by using microfluid catridges. While this is ideal for a end user point-of-care application, it is overkill in a research setting where experimentation is taking place. Therefore, the design could be simplified. The main issue is that there doesn't appear to be any off the shelf option that is affordable. Building a device could be done affordably but would require more time and access to a calibration device.
There are other options that are available off the shelf such as the minipcr. A starter kit can be purchased for $1250 that includes the PCR thermocycler, electrophoresis visualization system and miscellaneous lab supplies. However, this system being PCR has some disadvantages compared to LAMP as described above.

I am still doing a survey as there is a lot of information out there. Just wondering if anyone else has looked into this at all and had any input. Would anyone be interested in getting involved whether through help with research, funding, etc?
Some of the initial applications I am specifically interested in are:
- Pathogen detection/discovery (nucleic acid amplification, reverse transcriptase activity)
- Genetic testing (carrier status, traits, etc.)
There was an open source project done called the uBAR using LAMP.

The authors claim prototype units can be built for $919 in single unit quantities. The unit automates analysis by using microfluid catridges. While this is ideal for a end user point-of-care application, it is overkill in a research setting where experimentation is taking place. Therefore, the design could be simplified. The main issue is that there doesn't appear to be any off the shelf option that is affordable. Building a device could be done affordably but would require more time and access to a calibration device.
There are other options that are available off the shelf such as the minipcr. A starter kit can be purchased for $1250 that includes the PCR thermocycler, electrophoresis visualization system and miscellaneous lab supplies. However, this system being PCR has some disadvantages compared to LAMP as described above.

I am still doing a survey as there is a lot of information out there. Just wondering if anyone else has looked into this at all and had any input. Would anyone be interested in getting involved whether through help with research, funding, etc?
Some of the initial applications I am specifically interested in are:
- Retrovirus detection / discovery
- Medical marijuana yeast and mold detection
Last edited: