My understanding (which may not be correct) is that all the processes that supply energy to the Krebs cycle, in order to turn the wheels of the Krebs cycle, do so via creation of acetyl-CoA.
At least from my perspective, we may have a problem of semantics. I'd clarify that Acetyl-CoA can't supply energy per se; it just supplies itself at what is usually drawn at the 'top' of the Krebs cycle, and supplies its acetyl group to make citrate in the citric acid cycle. Acetyl Co-A does also help oxidize fatty acids in situations of lower glucose, so it is in part responsible for fat breakdown as well.
Acetyl CoA is even responsible for the production of cholesterol, which goes on to make hormones (which I'm sure you know
@Hip but I'm stating for the purposes of clarity here).

The only one where Acetyl CoA isn't absolutely required is the digestion of proteins.
Some amino acids break down into keto acids. Some will enter the cycle above pyruvate and therefore still 'pass through' Acetyl CoA. Others enter the cycle at oxaloacetate, further on in the cycle; and others enter at alpha-ketoglutarate, even further down.
I wouldn't say that Acetyl CoA isn't
involved because they're all part of a cycle, which means every part of the process 'leans' on every other part. But there are places for amino acids to enter the cycle that are after (or depending on your point of view, before) Acetyl CoA.
Acetyl-CoA, on the other hand, derived from pyruvate oxidation, or from the beta-oxidation of fatty acids, is the only fuel to enter the citric acid cycle.
I find that to be a weird statement in general. Note there's nothing about proteins, our other macronutrient. Proteins would not be a macronutrient if they didn't play a part in cellular respiration someplace.

This is from an explanation I made regarding (I think) C Armstrong's paper before last. You can see that amino acids enter the cycle at various points, though:
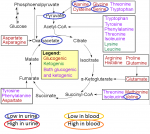
I also read that acetyl-CoA is the only molecule that gets used up by the Krebs cycle; whereas all Krebs cycle intermediates are not used up, but are converted from one to another
"Not used up" BUT "converted from one form into another".
Since Acetyl CoA is a molecule, the only way you can use it up is to convert it from one form to another. Since matter is never created or destroyed, this is the only way in which a molecule can be used up.
this means that if you replenish one of the Krebs cycle intermediates, such as say alpha-ketoglutarate, then you actually replenish all the intermediates, because the alpha-ketoglutarate you added to the Krebs cycle will get converted to every other intermediate.
Arguably the case, PROVIDED that all of the enzymes that help catalyze those reactions are present and accounted for, and not blocked by inhibitors; which F & M say is not the case.
However, as far as we know, only the pyruvate > acetyl-CoA conversion is inhibited in ME/CFS, but the pyruvate > oxaloacetate and the pyruvate > alpha-ketoglutarate replenishment conversions are still functional. So there should be plenty of pyruvate available for the purpose of replenishing the Krebs cycle intermediates.
Pyruvate to oxaloacetate requires that there first be pyruvate, which would be lower without Acetyl CoA.
Ditto for pyruvate to alpha-ketoglutarate.
You can feed amino acids straight to alpha-ketoglutarate in the cycle. But since, in a healthy person, you'd also be getting
additional alpha-ketoglutarate as the result of glucose metabolism, without proper glucose metabolism (and without increased amino acid uptake) you'd have lower alpha-ketoglutarate.
As you say, if you increase alpha-ketoglutarate you increase everything in the CAC (without errors in enzymes) -- but you have to have some way of increasing it! Women, at least, are feeding it allll their protein, according to F & M.
Note: all of this is upper-level-high-school knowledge and may be incomplete. Take with a grain of salt!
-J

