STUDY 1: Vagus nerve stimulation helps w/ autoimmune disorder
The Effects of Noninvasive Vagus Nerve Stimulation on Fatigue and Immune Responses in Patients With Primary Sjögren's Syndrome.
Tarn J1,
Legg S1,
Mitchell S1,
Simon B2,
Ng WF1.
Author information
Abstract
OBJECTIVES:
Primary Sjögren's syndrome (pSS) sufferers have rated chronic fatigue as the most important symptom needing improvement. Emerging data suggest that stimulation of the vagus nerve can modulate immunological responses. The gammaCore device (electroCore), developed to stimulate the cervical vagus nerve noninvasively, was used to assess the effects of vagus nerve activation on immune responses and clinical symptoms of pSS.
MATERIALS AND METHODS:
Fifteen female pSS subjects used the nVNS device twice daily a 26-day period. At baseline, blood was drawn before and after application of the gammaCore device for 90 sec over each carotid artery. The following fatigue-related outcome measures were collected at baseline, day 7 and day 28: EULAR patient reported outcome index, profile of fatigue (Pro-F), visual analogue scale of abnormal fatigue, and Epworth sleepiness scale (ESS). Whole blood samples were stimulated with 2 ng/mL lipopolysaccharide (LPS) and the supernatant levels of IFNγ, IL12-p70, TNFα, MIP-1α, IFNα, IL-10, IL-1β, IL-6, and IP-10 were measured at 24 hours. In addition, clinical hematology and flow cytometric profiles of whole blood immune cells were analyzed.
RESULTS:
Pro-F and ESS scores were significantly reduced across all three visits. LPS-stimulated production of IL-6, IL-1β, IP-10, MIP-1α, and TNFα were significantly reduced over the study period. Patterns of NK- and T-cell subsets also altered significantly over the study period. Interestingly, lymphocyte counts at baseline visit correlated to the reduction in fatigue score.
CONCLUSION:
The vagus nerve may play a role in the regulation of fatigue and immune responses in pSS and nVNS may reduce clinical symptoms of fatigue and sleepiness. However, a sham-controlled follow-up study with a larger sample size is required to confirm the findings
STUDY 2: MIcrobiome wipeout helps w/ autoimmune disorder
Oral antibiotics as a novel therapy for arthritis: evidence for a beneficial effect of intestinal Escherichia coli.
Nieuwenhuis EE1,
Visser MR,
Kavelaars A,
Cobelens PM,
Fleer A,
Harmsen W,
Verhoef J,
Akkermans LM,
Heijnen CJ.
Author information
Abstract
OBJECTIVE:
The intestinal flora is thought to play an important role in regulation of immune responses. We investigated the effects of changing the intestinal flora on the course of adjuvant-induced arthritis (AIA) and on experimental autoimmune encephalomyelitis (EAE) by the use of oral antibiotics.
METHODS:
Oral treatment with either vancomycin or vancomycin, tobramycin, and colistin was started after AIA and EAE induction. Clinical symptoms of AIA and EAE were monitored, and microbial analysis of ileal samples was performed.
RESULTS:
Oral vancomycin treatment after disease induction significantly decreased clinical symptoms of AIA. Simultaneously, increased concentrations of Escherichia coli were detected in the distal ileum of vancomycin-treated rats. Ileal concentrations of E coli were inversely related to disease scores in rats with AIA. Coadministration of colistin/tobramycin to prevent the increase in E coli abrogated the beneficial effect of vancomycin on AIA. Vancomycin treatment also reduced the clinical symptoms of EAE.
CONCLUSION:
We propose oral vancomycin as a novel therapeutic strategy in autoimmune diseases
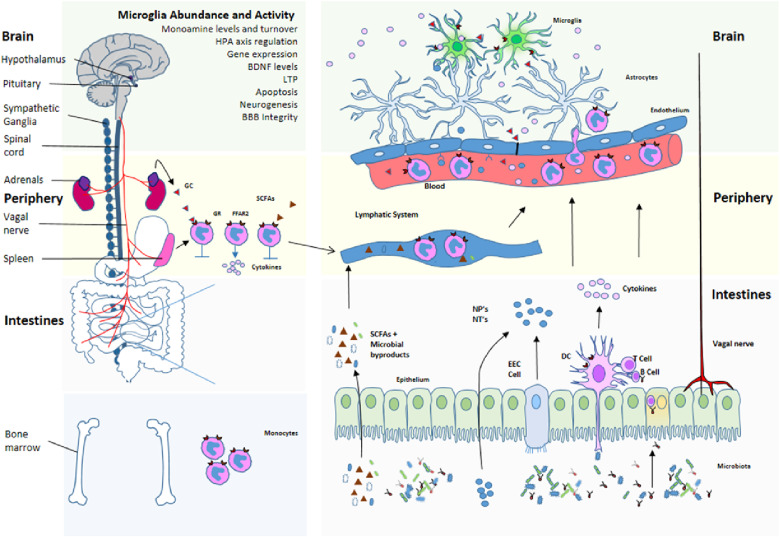
STUDY 3: Disbiosys negatively affect vagus nerve
The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis
Bruno Bonaz,1,2,*
Thomas Bazin,3,4 and
Sonia Pellissier5
Abstract
The microbiota, the gut, and the brain communicate through the microbiota-gut-brain axis in a bidirectional way that involves the autonomic nervous system. The vagus nerve (VN), the principal component of the parasympathetic nervous system, is a mixed nerve composed of 80% afferent and 20% efferent fibers. The VN, because of its role in interoceptive awareness, is able to sense the microbiota metabolites through its afferents, to transfer this gut information to the central nervous system where it is integrated in the central autonomic network, and then to generate an adapted or inappropriate response. A cholinergic anti-inflammatory pathway has been described through VN's fibers, which is able to dampen peripheral inflammation and to decrease intestinal permeability, thus very probably modulating microbiota composition. Stress inhibits the VN and has deleterious effects on the gastrointestinal tract and on the microbiota, and is involved in the pathophysiology of gastrointestinal disorders such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) which are both characterized by a dysbiosis. A low vagal tone has been described in IBD and IBS patients thus favoring peripheral inflammation. Targeting the VN, for example through VN stimulation which has anti-inflammatory properties, would be of interest to restore homeostasis in the microbiota-gut-brain axis