Ecoclimber
Senior Member
- Messages
- 1,011
There are some in the ME/CFS medical field that believe ME/CFS is 'MS Light' or 'Atypical MS'. The reason I post these articles is the fact that research in one area may spill over into another area of research or the fact that researchers reviewing a site may look at the research in another disease category that could be related to theirs and it might raise their interest level.
Futhermore, research may be further ahead in another field that ME/CFS researchers wish to explore if they had the funding and the researchers to explore such as EBV, HERVs, Autoimmune diseases, Fibromyalgia, Lyme etc.
Permission to post Prof. Permission to repost by Prof. G
I thought this was interesting discussion on the various MS-DMT used by reserchers in MS and might be helpful for researchers exploring rituxan for mecfs
CD20 antibodies deplete EBV
Futhermore, research may be further ahead in another field that ME/CFS researchers wish to explore if they had the funding and the researchers to explore such as EBV, HERVs, Autoimmune diseases, Fibromyalgia, Lyme etc.
Permission to post Prof. Permission to repost by Prof. G
I thought this was interesting discussion on the various MS-DMT used by reserchers in MS and might be helpful for researchers exploring rituxan for mecfs
CD20 antibodies deplete EBV
If you haven’t been living under a rock for the past few months  ,
,
you will have noticed that we have been saying that MS drugs that work the best, seem to deplete the memory B cells the most.
What! You are a ground hog and have just come out of hibernation.
Ok… go on have a read. Its free (Click Here). Let’s get that altmetric up:-0
Centre stage is the memory B cells. According to the Immunologist they can turn into antibody making machines to help create MS, they can make goodies (cytokines) or should I say baddies that can help other cells to create MS and for the die-hards stuck in the mud, they can stimulate T cells to do the business.
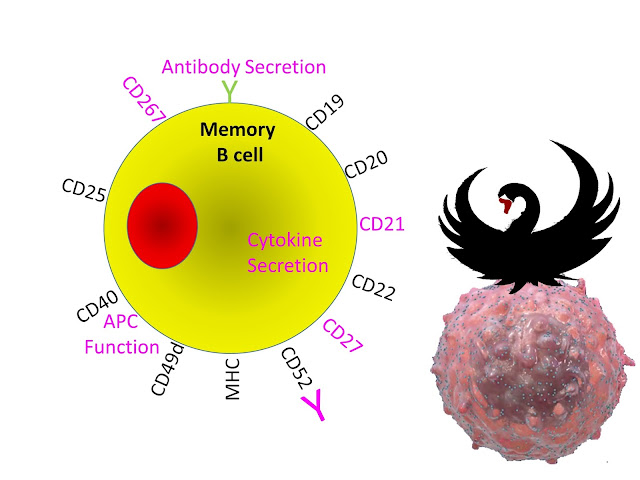
However, we can see ProfG’s Black swan perched on a Roche-inspired B cell. Don’t understand have a read of past posts.
http://multiple-sclerosis-research.blogspot.com/2013/09/what-does-black-swan-have-to-do-with-ms.html
https://www.blogger.com/goog_1274647104
http://multiple-sclerosis-research.blogspot.com/2013/09/key-ms-research-priorities.html
http://multiple-sclerosis-research.blogspot.com/2014/03/ebv-re-activation-in-relapses.html
But ProfG’s swan may be laying a big egg. The dots may not be CD20 the target for Ocrelizumab as Roche intended, but seeds of discontent for alternative thought and the blobs of budding virus.
http://multiple-sclerosis-research.blogspot.com/2017/03/clinicspeak-mode-of-action-of-b-cell.html
my favorite
http://multiple-sclerosis-research.blogspot.com/2017/03/what-did-b-cell-video-miss.html
Epstein Bar Virus infects via a B cell marker and hides in memory B cells. This shows itself every so often, when the T cells can kick it's butt.
So we suggested rather than depleting B cells to treat MS, ocrelizumab may be destroying a virus factory. Indeed we said that all active MS drugs may be doing this.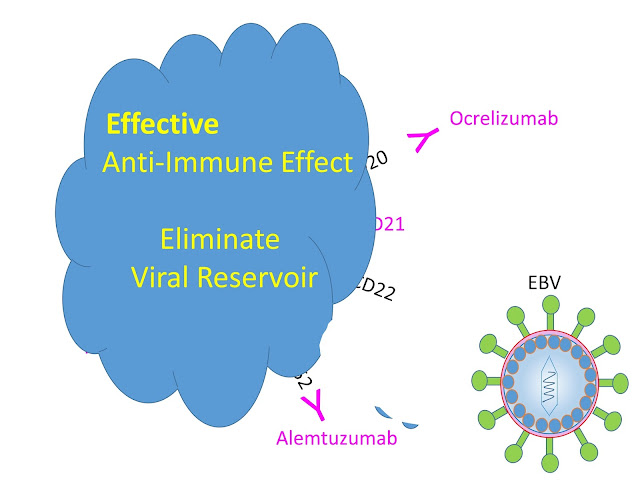
Is there evidence for this?
Do we do some work or just a bit of reading?
The following was not done in MS (I have removed the disease)
Magnusson M et al. Epstein–Barr virus in bone marrow of patients predicts response to rituximab treatment.
Objectives. Viruses may contribute to disease. This prompted us to monitor viral load and response to anti-CD20 therapy in patients.
Methods. Blood and bone marrow from 35 patients were analysed for CMV, EBV, HSV-1, HSV-2,parvovirus B19 and polyomavirus using real-time PCR before and 3 months after rituximab (RTX) treatment and related to the levels of autoantibodies and B-cell depletion. Clinical response to RTX was defined as decrease in the disease activity score (DAS) >1.3 at 6 months.
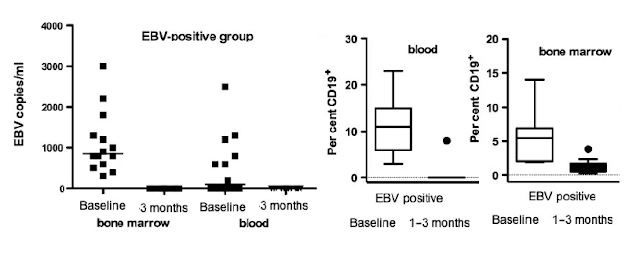
Results. Before RTX treatment, EBV was identified in 15 out of 35 patients (EBV-positive group), of which 4 expressed parvovirus. Parvovirus was further detected in eight patients (parvo-positive group). Twelve patients were negative for the analysed viruses. Following RTX, EBV was cleared, whereas parvovirus was unaffected. Eighteen patients were responders, of which 12 were EBV positive. The decrease in the DAS was significantly higher in EBV-positive group compared with parvo-positive group (P = 0.002) and virus-negative patients (P = 0.04). Most of EBV-negative patients that responded to RTX (75%) required retreatment within the following 11 months compared with only 8% of responding EBV-positive patients. A decrease of Ig-producing cells and CD19+ B cells was observed following RTX but did not distinguish between viral infections. However, EBV-infected patients had significantly higher levels of Fas (Suicide molecule) -expressing B cells at baseline as compared with EBV-negative groups.
Conclusions. EBV and parvovirus genomes are frequently found in bone marrow of patients. The presence of EBV genome was associated with a better clinical response to RTX. Thus, presence of EBV genome may predict clinical response to RTX.
So what does this study show. You can have a look at a few graphs from the paper.
EBV was eliminated from by the treatment of rituximab. This would be consistent with the B cells being infected by EBV being depleted.
Those with the highest level of EBV showed the best response to therapy, compared to people who were so-called EBV negative (This proportion is too low compared to what we know of the normal population).
Anyway, I have suggested that looking at memory B cells may be able to predict response to therapy, but this shows a hole in the argument because you can see that rituximab emptied the blood of B cells and as memory B cells make up about 30% of the CD19 population they are emptied from the blood too, and in this case there was not a 100% response to therapy.
However, if we drive from London (Bone Marrow/Lymph gland) to Leeds (Brain) along the M1 motorway (blood) and we can see a car (B cell) if we have a look for a minute from a bridge over the motorway (blood test). If I drive my car (Pathogenic B cell) to York (Centre of Gods own Country about 200miles north) to cause the problem (MS), if you look at 3 in the afternoon you might see loads of cars but do it at 3 in the morning and you might not see any although the problem is indeed a car driving to York (a few miles from Leeds) along the M1 motorway.
The other problem is the treatment failure may be because the cells causing the failure are already in the brain and it is too late. In the ocrelizumab trials they re-baselined their results to allow 3 months
for the drug to work. A sensible thing to do if you are looking for efficacy.
However, you can also see that the bone marrow was not emptied by rituximab. If we look at other posts of rituximab, we can see that the lymph glands are not effectively depleted.
Subcutaneous versus Intravenous Administration of Rituximab: Pharmacokinetics, CD20 Target Coverage and B-Cell Depletion in Cynomolgus Monkeys Cheng-Ping Mao, Martin R. Brovarney, Karim Dabbagh, Herbert F. Birnböck, Wolfgang F. Richter, Christopher J. Del Nagro PLoS One. 2013; 8(11): e80533
Similarly, levels of peripheral blood B cells were depleted by >94% for both subcutaneous and intravenous dosing. B-cell levels were decreased by 57% (subcutaneous) and 42% (intravenous...so not that great) respectively. Yes this is in monkies but I could be bothered to go through 4000 references to pick a relevant human one.
Looks like ocrelizumab does something similar
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761053Orig1s000PharmR.pdf
Now if you remember the monday post
http://multiple-sclerosis-research.blogspot.com/2017/05/what-is-your-choice.html
where it was suggested that alemtuzumab may fail in many people after switching from fingolimod
http://multiple-sclerosis-research.blogspot.com/2017/01/fingolimod-blocking-alemtuzumab-action.html
This is because it traps white blood cells in the lymph glands and bone marrow and because alemtuzumab does not clear out the bone marrow (at least in animals expresing human CD52). Is the level of purging of the bone marrow and lymph glands by ocrelizumab going to be enough so it doesn't to suffer the same fate as alemtuzumab after fingolimod treatment?
Posted by MouseDoctoryou will have noticed that we have been saying that MS drugs that work the best, seem to deplete the memory B cells the most.
What! You are a ground hog and have just come out of hibernation.
Ok… go on have a read. Its free (Click Here). Let’s get that altmetric up:-0
Centre stage is the memory B cells. According to the Immunologist they can turn into antibody making machines to help create MS, they can make goodies (cytokines) or should I say baddies that can help other cells to create MS and for the die-hards stuck in the mud, they can stimulate T cells to do the business.
However, we can see ProfG’s Black swan perched on a Roche-inspired B cell. Don’t understand have a read of past posts.
http://multiple-sclerosis-research.blogspot.com/2013/09/what-does-black-swan-have-to-do-with-ms.html
https://www.blogger.com/goog_1274647104
http://multiple-sclerosis-research.blogspot.com/2013/09/key-ms-research-priorities.html
http://multiple-sclerosis-research.blogspot.com/2014/03/ebv-re-activation-in-relapses.html
Search on "Black Swan"But ProfG’s swan may be laying a big egg. The dots may not be CD20 the target for Ocrelizumab as Roche intended, but seeds of discontent for alternative thought and the blobs of budding virus.
http://multiple-sclerosis-research.blogspot.com/2017/03/clinicspeak-mode-of-action-of-b-cell.html
my favorite
http://multiple-sclerosis-research.blogspot.com/2017/03/what-did-b-cell-video-miss.html
Epstein Bar Virus infects via a B cell marker and hides in memory B cells. This shows itself every so often, when the T cells can kick it's butt.
So we suggested rather than depleting B cells to treat MS, ocrelizumab may be destroying a virus factory. Indeed we said that all active MS drugs may be doing this.
Is there evidence for this?
Do we do some work or just a bit of reading?
The following was not done in MS (I have removed the disease)
Magnusson M et al. Epstein–Barr virus in bone marrow of patients predicts response to rituximab treatment.
Objectives. Viruses may contribute to disease. This prompted us to monitor viral load and response to anti-CD20 therapy in patients.
Methods. Blood and bone marrow from 35 patients were analysed for CMV, EBV, HSV-1, HSV-2,parvovirus B19 and polyomavirus using real-time PCR before and 3 months after rituximab (RTX) treatment and related to the levels of autoantibodies and B-cell depletion. Clinical response to RTX was defined as decrease in the disease activity score (DAS) >1.3 at 6 months.
Results. Before RTX treatment, EBV was identified in 15 out of 35 patients (EBV-positive group), of which 4 expressed parvovirus. Parvovirus was further detected in eight patients (parvo-positive group). Twelve patients were negative for the analysed viruses. Following RTX, EBV was cleared, whereas parvovirus was unaffected. Eighteen patients were responders, of which 12 were EBV positive. The decrease in the DAS was significantly higher in EBV-positive group compared with parvo-positive group (P = 0.002) and virus-negative patients (P = 0.04). Most of EBV-negative patients that responded to RTX (75%) required retreatment within the following 11 months compared with only 8% of responding EBV-positive patients. A decrease of Ig-producing cells and CD19+ B cells was observed following RTX but did not distinguish between viral infections. However, EBV-infected patients had significantly higher levels of Fas (Suicide molecule) -expressing B cells at baseline as compared with EBV-negative groups.
Conclusions. EBV and parvovirus genomes are frequently found in bone marrow of patients. The presence of EBV genome was associated with a better clinical response to RTX. Thus, presence of EBV genome may predict clinical response to RTX.
So what does this study show. You can have a look at a few graphs from the paper.
EBV was eliminated from by the treatment of rituximab. This would be consistent with the B cells being infected by EBV being depleted.
Those with the highest level of EBV showed the best response to therapy, compared to people who were so-called EBV negative (This proportion is too low compared to what we know of the normal population).
Anyway, I have suggested that looking at memory B cells may be able to predict response to therapy, but this shows a hole in the argument because you can see that rituximab emptied the blood of B cells and as memory B cells make up about 30% of the CD19 population they are emptied from the blood too, and in this case there was not a 100% response to therapy.
However, if we drive from London (Bone Marrow/Lymph gland) to Leeds (Brain) along the M1 motorway (blood) and we can see a car (B cell) if we have a look for a minute from a bridge over the motorway (blood test). If I drive my car (Pathogenic B cell) to York (Centre of Gods own Country about 200miles north) to cause the problem (MS), if you look at 3 in the afternoon you might see loads of cars but do it at 3 in the morning and you might not see any although the problem is indeed a car driving to York (a few miles from Leeds) along the M1 motorway.
So we may have to look outside of the blood to get the answer
The other problem is the treatment failure may be because the cells causing the failure are already in the brain and it is too late. In the ocrelizumab trials they re-baselined their results to allow 3 months
for the drug to work. A sensible thing to do if you are looking for efficacy.
However, you can also see that the bone marrow was not emptied by rituximab. If we look at other posts of rituximab, we can see that the lymph glands are not effectively depleted.
Subcutaneous versus Intravenous Administration of Rituximab: Pharmacokinetics, CD20 Target Coverage and B-Cell Depletion in Cynomolgus Monkeys Cheng-Ping Mao, Martin R. Brovarney, Karim Dabbagh, Herbert F. Birnböck, Wolfgang F. Richter, Christopher J. Del Nagro PLoS One. 2013; 8(11): e80533
Similarly, levels of peripheral blood B cells were depleted by >94% for both subcutaneous and intravenous dosing. B-cell levels were decreased by 57% (subcutaneous) and 42% (intravenous...so not that great) respectively. Yes this is in monkies but I could be bothered to go through 4000 references to pick a relevant human one.
Looks like ocrelizumab does something similar
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761053Orig1s000PharmR.pdf
Now if you remember the monday post
http://multiple-sclerosis-research.blogspot.com/2017/05/what-is-your-choice.html
where it was suggested that alemtuzumab may fail in many people after switching from fingolimod
http://multiple-sclerosis-research.blogspot.com/2017/01/fingolimod-blocking-alemtuzumab-action.html
This is because it traps white blood cells in the lymph glands and bone marrow and because alemtuzumab does not clear out the bone marrow (at least in animals expresing human CD52). Is the level of purging of the bone marrow and lymph glands by ocrelizumab going to be enough so it doesn't to suffer the same fate as alemtuzumab after fingolimod treatment?