Wow
@Kimsie, that is some dense reasoning! (Dense like a fudge cake, not like "bad.")
I am not a biochemist or any such, but I do have a question about part of your theory: You suggest that as we become low on thiamine we would start to lose out on NADPH, and that the folate cycle would basically creatively contort itself in a cycle that produces NADPH and ammonia, but uses up serine. My question is about serine: As I understand it, we can synthesize serine (and in a case like this where we are using more of it, I will assume that we are synthesizing it), and serine synthesis uses an ammonia donated from glutamate. So it seems like the reaction you have described--which still seems like a theoretically good way raise nadph--would not end up producing excess ammonia in the long run. Although perhaps in the short run?
Of course, then there is the question of why do people with chronic fatigue generally have low NADPH if this cycle can come into play? Is it blocked in some way? Is something else limiting it? Is serine synthesis limited in some way?
You know, the glycine decarboxylase complex might account for why Rich Van K found low glycine in a lot of people with chronic fatigue. Maybe it was all eaten up to provide more NADPH! (Or else synthesis was blocked along with the rest of the folate cycle, and then we supplemented lots of MTHF, which bypassed the need to move THF to methylene THF, thus preventing glycine synthesis.)
I hope you continue to let us know what you find out. It would be very interesting if B2, by using up active B1, was increasing ammonia somehow.
And obviously, perhaps I have missed something somewhere.
What do you think?
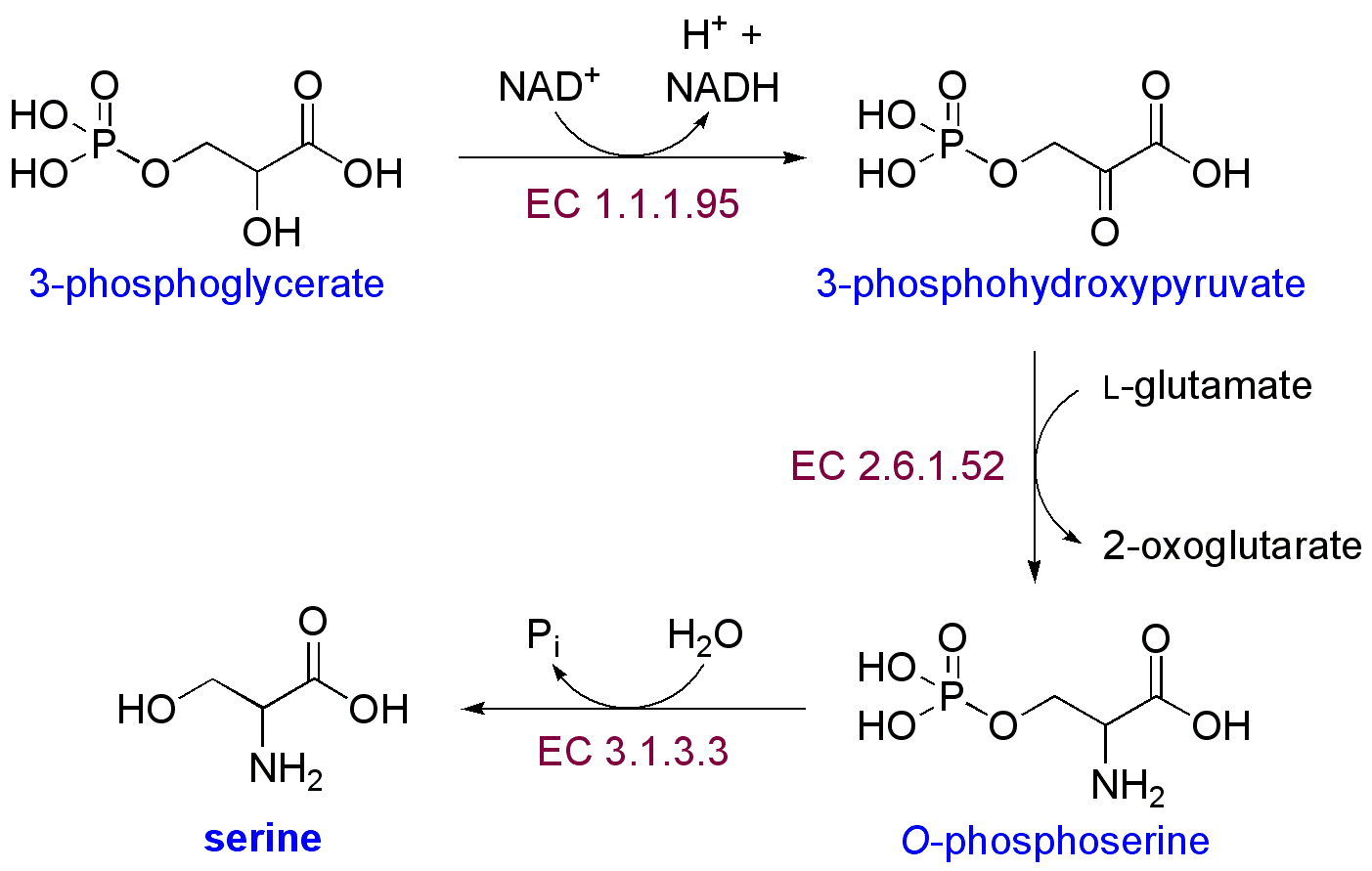
What I put into the post in this thread isn't really complete without some other information about what I have been learning in the past few months. I am pretty sure that there are some blockages in the glycolysis pathway and the availability of pyruvate is more limited in a lot of people with certain chronic illnesses than in healthy people. I theorize that the reason for this is because there is a problem with sulfite oxidase and sulfite levels are higher than normal and the sulfite is causing problems. If a person does not have any problem with this particular pathway to make serine, then using serine and glycine for NADPH synthesis should not create a problem with ammonia. This is how the pathway is
supposed to work.
Here is an interesting study where they found that people with all kinds of chronic illnesses, including some with CFS, had a
high cysteine/sulfate ratio, and I think that this must be caused by a problem with sulfite oxidase. Unfortunately, they didn't check the sulfite/cysteine ratio in this study, because I think that is what causes the problem, if I am right. Here is a study which talks about how sulfite
interferes with glycolysis. So the point of this is that sulfite interferes with glycolysis in at least two places, and it could interfere in the pathway for serine synthesis that you mention because 3-phosphoglycerate comes from that pathway. Another possible problem with making serine from this pathway is that it uses an aminotransferase and all aminotransferases requires B6, so if a person is low in B6, they could have problems making serine in this way. That could be another reason why B2 can cause depression, because the SHMT can drain B6 and the aminotransferases are vital to neurotransmitter function. I can think of a lot of possible ways that B2 could cause depression, and they all involved an imbalance of B vitamins. I have researched this a lot because of my son with depression.
The reason I hypothesize that this process is causing more ammonia to be released is because the urine amino acids are so low, and so they must be being broken down more than they would have been normally, which releases ammonia. (My husband was eating a diet quite high in animal protein when his urine tested so low in amino acids, so his levels were not low from low dietary intake.)
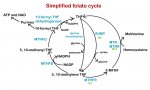
The folate cycle isn't contorting itself when it makes NADPH. It's normal for the pathway to run that way, (see the article I mention in my post above). Probably most people here haven't taken a good look at the whole folate cycle; the version that is seen a lot, that Amy Yasko uses, is super simplified and makes it look like the folate cycle is simpler than the methionine cycle, when it is actually about 10 times more complicated than the methionine cycle and it has a lot of loops going different ways. Making methionine is just one of the many important things that folate does. Most likely, in a healthy person, only excess serine and glycine is used in this pathway.
Another pathway to make serine and glycine is from malate, which can be used to make pyruvate, and the malate can come from the amino acids which can enter the TCA cycle as intermediates, i.e. glutamine, histidine, proline, arginine, valine, isoleucine, methionine, tyrosine, phenylalanine, asparagines, and aspartate. The other amino acids, except taurine, can mostly enter the TCA cycle as acetyl CoA. So the loss of amino acids could be mainly from the use of amino acids to fuel the TCA cycle because glycolysis is inhibited, and it might not have anything to do with low thiamine. However, the difference with ammonia in the sweat of my husband gives support to the idea that at least in his case, his amino acids are being used to make serine/glycine for this pathway. Actually, now that I think about it, it isn't that ammonia is released particularly in the glycine reaction that would cause the higher ammonia, it is that amino acids are being catabolized (broken down) that would not be broken down other wise. With more thiamine my husband doesn't catabolize as much protein and he has less ammonia. It was really cute, while I was writing this he came in and held out his wet shirt for me to smell; he just can't get over the difference!
It might be that some people are having difficulty with this serine/glycine pathway and so can't make enough NADPH even with the alternative pathway. This is probably the case with a lot of CFS people because I think they are low in a lot of cofactors. This alternative pathway requires not only folate, but B6 also, so low B6 will inhibit this pathway at SHMT. In fact, part of my hypothesis is that in some people with chronic illness, this pathway causes people to lose B6. This is what happened to my son with depression - about a month after he started taking a lot of folate he started getting depressed, but before he started the folate he had terrible fatigue. I think that if we had been giving large doses of B6 and thiamine (and he was taking 50 mg of P5P but that wasn't enough) along with the folate and other vitamins he was taking, he would not have become depressed.
If a person knows that they are low in NADPH, then I think that they may want to try taking a pretty large dose of thiamine, but not without the other B's. Our experience is that a much lower dose of riboflavin is needed than thiamine, so I can see why taking B2 without thiamine caused a problem. There could be some people who need more riboflavin, though.
Supplementing with a lot of folate wouldn't stop any pathway from going, except if it caused another vitamin to be drained, such as I think it can cause a drain of B6 in certain instances, as I mentioned above. In these pathways each molecule of folate (this is true with other cofactors also) is used over and over many thousands of times, and the pathway is not regulated by the availability of folate, but by other things such as the amount of substrate and product (what the enzyme works on and what it produces), etc. A shortage of a cofactor will inhibit a pathway, and so people seem to draw the conclusion that an abundant amount of a cofactor will do the opposite and make too much of something, but this can only happen for a short while until the body adjusts the levels of the enzymes. For instance, in the case of MTHFR high levels of SAMe inhibit MTHFR in just a few minutes. Other enzymes might take a few hours to be adjusted. Some take a few days.
If a vitamin causes symptoms immediately, then I think that is a sign that another vitamin is lacking, such as when a person gets anxiety from taking a large dose of folate - because they don't have enough niacin. If it takes more time, then probably another vitamin is getting drained and one can look at the pathways involved to try to figure out which one it might be, such as in the case with B1 and B2 being used together in several very important enzymes. Another cause of symptoms is that, in chronic illness, often glutathione and other toxin clearing pathways are being affected and sometimes taking vitamins improves these toxin clearing pathways and the person can have nausea and other symptoms associated with detoxification.