I've written on this paper so many times now that I'm beginning to think it should be called Jaime's Bane, like the Ring of Power or something. I have literally written to the researchers and informed them of a mistake in their data, that's how long I've been looking at this thing. Which means that I knew that I'd better finish this now or I'd end up casting it into the pit at Mount Doom instead, otherwise known as saying, "I know I told everyone I'd do a write-up, but surely everyone's forgotten all that by now..." 
 HAHAHAno.
HAHAHAno.
So we need some background in order to understand the pertinence of everything being discussed here, and frankly I really needed a refresher on this - it's been a long time since freshman biology! So I colored you guys some pretty pictures, and re-educated myself in the process.
Cellular Respiration:
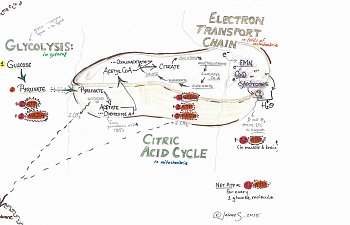
This is a picture of aerobic respiration in the cell:
The thing that looks like half a bean is a mitochondria, where the vast majority of your energy molecules are produced.
Glycolysis:
Glycolysis does not occur in the mitochondria, but in the cytosol (still inside the cell, but just floating around in the cytoplasm) and is the only anaerobic part of the process, meaning it does not require oxygen to work. Despite the way I've depicted it here, it's actually a multi-step process starting with glucose and involving catalysis by a fistful of enzymes to eventually produce pyruvate. If you would like to see glycolysis in all of its glory, follow the link to the glycolysis wiki and hover over the image to the right; it will tell you the names of each enzyme involved every step of the way.
Glycolysis is does not produce a lot of our energy molecules: only 2 ATP out of 36/38 ATP. Still, since it's the first step, it 'feeds' pyruvate into the citric acid cycle, which makes it very important: it's the first in a long line of dominoes we have to tip to get as much energy as possible. As to why ATP, NADH, and FADH are important molecules for energy, read on!
The pyruvate produced in glycolysis enters the mitochondria and is oxidized to acetate, which then reacts with coenzyme A.
We require Vitamin B5 to produce a good amount of coenzyme A, so for some ME /CFSers, this might be your rate-limiting step.
When acetate and coenzyme A react, they make Acetyl Co-A (or 'acetyl coenzyme A'), which enters the citric acid cycle.
Summary of the steps after glycolysis but before the citric acid cycle begins:
Citric Acid Cycle:
Acetyl CoA then reacts with oxaloacetate to produce citrate, which loses some carbons and produces 2 more ATP molecules (same number as last part of the cycle). Now, over the course of this cycle, we're accumulating electrons. These electrons are usually 'held' by an acceptor molecule like FADH2 or NADH. This is why these molecules are important for energy, because next they carry those extra electrons to the third and final major process in aerobic respiration:
Electron Transport Chain:
Think of this one like a bucket brigade.
Each chemical on the electron transport chain is passing electrons down the line, starting with energy molecules like NADH and FADH2. Several members of this chain of 'bucket brigade' chemicals include flavin mononucleotide (FMN, from B2), Coenzyme Q-10, and a bunch of the cytochromes. Sounding familiar? All of these chemicals have a form that has fewer electrons and a form that has more, and they shift back and forth as they accept electrons before handing them down to the next guy along the chain.
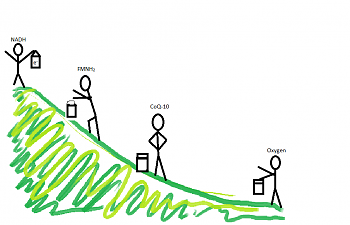
Each handoff requires less energy than the one before, until finally oxygen is reached. Visually, I think of the bucket brigade being on a hill, with NADH and FADH2 standing at the top, and oxygen at the bottom:
This can present another wrench in the gears. If the guy at the bottom of the 'hill' isn't there to take the accumulated electrons, guess what? There's a backup like a traffic jam, allll the way back to the very first electron donors, NADH and FADH2.
This could be a serious problem, because the chain generates a whopping 32-34 ATP molecules, and is where aerobic organisms like us get most of their energy. In fact, it's actually a tougher process in muscle and brain cells, where you can do more chemical 'work' without getting as much energy payback.
If all goes well however, the oxygen combines with some hydrogen and leaves the mitochondria as water.
Whew!
View attachment 11437
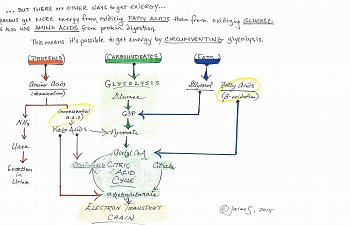
What I hope you can tell by looking at the diagram is that it's possible to circumvent glycolysis entirely, still getting nearly the right amount of energy by eating a diet high in protein and fatty acids. Believe it or not, you need to know that too, in order to understand the thrust of the article.
Okay, so the title of the paper is:
Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients
Christopher W. Armstrong, Neil R. McGregor, Donald P. Lewis, Henry L. Butt, and Paul R. Gooley, in Metabolomics, 22 May 2015
The Experimental Design:
Armstrong et al. wanted to determine what, if anything, was metabolically 'off' in ME / CFS patients. In order to determine this, the researchers recruited 34 females with ME/CFS (34.9+/- 1.6 SE y.o.) and 25 non-ME/CFS females (33.0 +/- SE y.o.) for purposes of comparison. Other participants were ruled out, including male patients with ME/CFS (sorry guys!) and people who were outliers age-wise. The dx criteria used was the Canadian Consensus; the non-ME/CFS patients were examined and it was determined they were not suffering from any major illness.
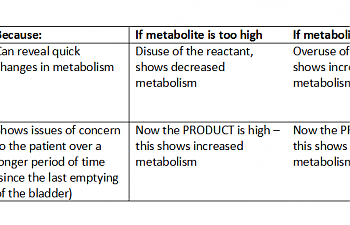
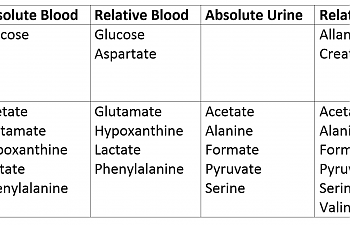
Then, they collected blood and urine from ill patients and healthy controls. In both cases, they then interpreted that data in two ways: by simply measuring the metabolites in the urine straight up, or doing a comparison between that metabolite and others in the same sample. This led to four separate values for both ME / CFS patients and healthy controls:
In order to understand the thrust of this article, one must keep in mind that the stuff in the urine should be 'used up', end-product-y stuff. If end products in the urine are high, that means that whatever cycle or process created them is on overdrive in the body.
It's kind of the opposite way for the blood; if something is high in the blood, it's probably not being used up very rapidly. Keep this in mind as we progress forward. If you like charts better:
View attachment 11435
Results:
Okay, guys, let's cut straight to the chase:
So, let's take a look at what this is telling us at the most basic level.
1) ME/CFS patients have hyperglycemia.
Glucose is high - both higher than in controls (absolute value) and high in comparison to other metabolites (relative value). This is saying in no uncertain terms that CFS / ME patients are hyperglycemic. It's a bold statement, actually, and ties in neatly with our neurological issues.
It also ties in with a recent study showing that, when biopsied muscle samples of ME / CFS patients are given an electric shock, the glucose in the muscle tissue does not decrease. However, that same glucose is responsive to insulin. In fact, the only reason that glucose would be in the muscle cells in any reasonable quantity is if insulin escorted it there.
This would mean that, instead of having a problem with our insulin like diabetics, we have a problem with glycolysis itself.
2) ME/CFS patients have signs of reduced glycolysis.
Look at how low pyruvate is in the urine. We are NOT flushing that stuff out; we're using it up like there's no tomorrow. That implies we're 'pushing' whatever pyruvate we DO have into the citric acid cycle. The same goes for acetate, which is oxidized pyruvate. These are the compounds that are produced 'between' glycolysis and the CAC, and the moment we get them, we're converting them to acetyl CoA and revving that Citric Acid Cycle engine as high as it'll go. The same goes for alanine and serine, which are also decreased in the urine of ME / CFS patients.
Lactate was low in blood, both relative and absolute. Shocking, you may say, given high lactate in some patients.
However, it makes sense with the reduced pyruvate. If glycolysis is scaled back, lactate, as a product of glycolysis, should be low as well. Keep in mind that this is not an exercise test, and these patients were just getting their blood drawn.
3) There are signs that other energy systems are trying to compensate.
GLUTAMATE:
The Citric Acid Cycle appears more or less intact, but a decrease in the concentrations of certain amino acids implies that they may be used to feed through the CAC, particularly glutamate, which is significantly reduced in the blood (again, showing over-utilization). Going by Hornig's two-phases hypothesis and my own experiences, however, this isn't surprising. It's quite possible that there is an excitatory initial phase of ME (with higher glutamate) and an immunosuppressive ME that presents later (with lower glutamate). (If GABA and NAC help you, high glutamate might be the culprit. If GABA and NAC made you feel weird or awful, there is a possibility that your glutamate is already low.)
Aspartate aminotransferase, which helps convert glutamate to aspartate, is elevated, as is aspartate itself. Thus, you get:
GLUTAMATE -----(Aspartate aminotransferase)---> ASPARTATE
It takes a lot of Vitamin B6 to push this reaction. Part of the reason why some of us might keel over without it could be that this is one of the compensatory energy mechanisms we're using to try to get enough without glycolysis working properly.
Low glutamate has been directly linked to diabetes and insulin resistance, although insulin resistance would appear to contradict the Newton paper showing muscle cell sensitivity to insulin.
PHENYLALANINE:
Perhaps most familiar to us because of the warning on every diet soda wrapper, phenylalanine is an amino acid that can be converted to tyrosine, which is the lead amino acid that helps us produce melatonin, thyroid hormones, serotonin, and a whole bunch of other stuff. But guess what? The reaction yields an NADP molecule, another energy molecule... so it's pushed. I'm actually a little surprised that tyrosine isn't high, but not that surprised. Given the stress the system is under in ME and CFS patients, that tyrosine may be snatched up to produce a hormone molecule the moment it appears.
CREATININE:
The creatinine being increased in the urine of ME / CFS patients implies that this reaction is being pushed. The phosphorylation of creatine isn't discussed much in Intro to Bio textbooks because it's not important in all tissues, but it is important in skeletal muscle, the heart, and the brain, where it makes ATP available super-quickly on demand - none of this three extensive, mad cycles nonsense. Think of the creatine kinase-creatinine-phosphorylcreatine system like a bank account with an ATM where you can get your energy super-quick, as opposed to investing in futures and waiting for the energy to come in (aerobic respiration!)
I've actually had this test come back a little high and been worried, because all I could find about high creatinine was kidney failure. However, it once again comes down to energy, and to us getting as much energy as we can as fast as we can grab hold of it.
This, as a mechanism, may explain why our muscles are so fatigued - we're 'borrowing' from that account with the ATM in order to pay the bills sent by our brain and heart until the muscles are bankrupt of energy, and we can't make any more withdrawals. (In fact, if we try, we may find we develop a service charge - a negative balance!)
This also may help explain why glycine is so low, because glycine makes creatine, which fuels this reaction.
Perhaps most convincingly, creatinine in the urine and glucose in the blood are directly proportional: the higher the blood sugar, the more creatinine in the ME / CFS patient's pee. This certainly implies that people who use glycolysis less need to use this pathway more.
HYPOXANTHINE and ALLANTOIN
It took me awhile to recall where I even knew this chemical from. I am very proud, because my ME-fogged-brain remembered that the answer was 'in your graduate thesis'. We had to chose an herb and outline its traditional uses, modern research, its chemistry, details of its value and how it was sold... and I, out of some puckish impulse, chose dandelion.
It didn't turn out to be a bad choice. There was oodles of information, and it was really interesting to write about something so common, but so useful.
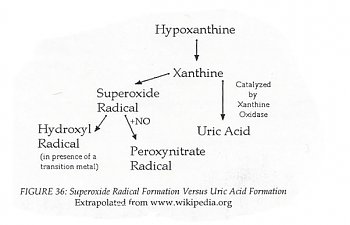
In any case, one of the papers I read showed that dandelion flower specifically scavenged the free radicals created by converting xanthine:
View attachment 11446
This means that if hypoxanthine is reduced in the blood, then this reaction is being 'pushed'.
Weirdly, I also know allantoin through plants: it is a chemical component of comfrey (Symphytum officinale), and is considered wound-healing. It's also the product of a reaction of uric acid in certain circumstance. Since hypoxanthine is at the top of the cycle and low, it makes sense that allantoin is an end-product, and high. Once again, this reaction generates NADH, so it is an energy reaction, though I feel like I understand this one the least. Xanthine and hypoxanthine are also part of an amino acid 'scavenging' reaction which I feel is important, but I think my brain is full, now.
Since this reaction generates some serious free radicals, this may be (part of) the source of the ME / CFS patient's over-oxidation.
I recommend dandelion flower tea to mop up the peroxynitrate and hydroxy radicals. Its effect is amplified by taking it with Vitamin E, as little!me read and faithfully reported about a decade ago (thanks, little me - who knew it would come in so handy?)
Limitations of the study:
It's important to be aware that this is quite a small study that, while it suggests directions for future research, does not provide strong support for any argument. A meta-analysis is in order specifically in the case of glucose, where Armstrong et al. tell us there are several other studies that agree with their findings, but were too small to draw conclusions. A meta-analysis is when a researcher takes the data from several other studies and combines them, resulting in a larger number of subjects, and therefore more meaningful results.
Another limitation is that all of the patients came from the same physician. This unfortunately increases the chances of homogeneity amongst the participants - could they have suffered from the same pathogenic 'initial insult'? Does this doctor have a narrower image of what constitutes 'true' ME, making their issues more similar than usual, patient to patient?
Finally, it all wraps things up a bit neatly for my taste. However, it also makes a lot of sense.
Advice that Would Make Sense in the Context of this Article:
What I'm really wondering, though, is when we get to start calling it Type III Diabetes....
-Jaime
View attachment 11433
One research paper to bring them all, and in the Darkness, Bind them...
("Mount Doom" by Source. Licensed under Fair use via Wikipedia - http://en.wikipedia.org/wiki/File:Mount_Doom.jpg#/media/File:Mount_Doom.jpg)
One research paper to bring them all, and in the Darkness, Bind them...
("Mount Doom" by Source. Licensed under Fair use via Wikipedia - http://en.wikipedia.org/wiki/File:Mount_Doom.jpg#/media/File:Mount_Doom.jpg)
So we need some background in order to understand the pertinence of everything being discussed here, and frankly I really needed a refresher on this - it's been a long time since freshman biology! So I colored you guys some pretty pictures, and re-educated myself in the process.
Cellular Respiration:
This is a picture of aerobic respiration in the cell:
The thing that looks like half a bean is a mitochondria, where the vast majority of your energy molecules are produced.
Glycolysis:
Glycolysis does not occur in the mitochondria, but in the cytosol (still inside the cell, but just floating around in the cytoplasm) and is the only anaerobic part of the process, meaning it does not require oxygen to work. Despite the way I've depicted it here, it's actually a multi-step process starting with glucose and involving catalysis by a fistful of enzymes to eventually produce pyruvate. If you would like to see glycolysis in all of its glory, follow the link to the glycolysis wiki and hover over the image to the right; it will tell you the names of each enzyme involved every step of the way.
Glycolysis is does not produce a lot of our energy molecules: only 2 ATP out of 36/38 ATP. Still, since it's the first step, it 'feeds' pyruvate into the citric acid cycle, which makes it very important: it's the first in a long line of dominoes we have to tip to get as much energy as possible. As to why ATP, NADH, and FADH are important molecules for energy, read on!
The pyruvate produced in glycolysis enters the mitochondria and is oxidized to acetate, which then reacts with coenzyme A.
We require Vitamin B5 to produce a good amount of coenzyme A, so for some ME /CFSers, this might be your rate-limiting step.
When acetate and coenzyme A react, they make Acetyl Co-A (or 'acetyl coenzyme A'), which enters the citric acid cycle.
Summary of the steps after glycolysis but before the citric acid cycle begins:
PYRUVATE ----> ACETATE + COENZYME A ---> ACETYL CO-A
Citric Acid Cycle:
Acetyl CoA then reacts with oxaloacetate to produce citrate, which loses some carbons and produces 2 more ATP molecules (same number as last part of the cycle). Now, over the course of this cycle, we're accumulating electrons. These electrons are usually 'held' by an acceptor molecule like FADH2 or NADH. This is why these molecules are important for energy, because next they carry those extra electrons to the third and final major process in aerobic respiration:
Electron Transport Chain:
Think of this one like a bucket brigade.
Each chemical on the electron transport chain is passing electrons down the line, starting with energy molecules like NADH and FADH2. Several members of this chain of 'bucket brigade' chemicals include flavin mononucleotide (FMN, from B2), Coenzyme Q-10, and a bunch of the cytochromes. Sounding familiar? All of these chemicals have a form that has fewer electrons and a form that has more, and they shift back and forth as they accept electrons before handing them down to the next guy along the chain.
Each handoff requires less energy than the one before, until finally oxygen is reached. Visually, I think of the bucket brigade being on a hill, with NADH and FADH2 standing at the top, and oxygen at the bottom:
This can present another wrench in the gears. If the guy at the bottom of the 'hill' isn't there to take the accumulated electrons, guess what? There's a backup like a traffic jam, allll the way back to the very first electron donors, NADH and FADH2.
This could be a serious problem, because the chain generates a whopping 32-34 ATP molecules, and is where aerobic organisms like us get most of their energy. In fact, it's actually a tougher process in muscle and brain cells, where you can do more chemical 'work' without getting as much energy payback.
If all goes well however, the oxygen combines with some hydrogen and leaves the mitochondria as water.
Whew!
View attachment 11437
What I hope you can tell by looking at the diagram is that it's possible to circumvent glycolysis entirely, still getting nearly the right amount of energy by eating a diet high in protein and fatty acids. Believe it or not, you need to know that too, in order to understand the thrust of the article.
Okay, so the title of the paper is:
Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients
Christopher W. Armstrong, Neil R. McGregor, Donald P. Lewis, Henry L. Butt, and Paul R. Gooley, in Metabolomics, 22 May 2015
The Experimental Design:
Armstrong et al. wanted to determine what, if anything, was metabolically 'off' in ME / CFS patients. In order to determine this, the researchers recruited 34 females with ME/CFS (34.9+/- 1.6 SE y.o.) and 25 non-ME/CFS females (33.0 +/- SE y.o.) for purposes of comparison. Other participants were ruled out, including male patients with ME/CFS (sorry guys!) and people who were outliers age-wise. The dx criteria used was the Canadian Consensus; the non-ME/CFS patients were examined and it was determined they were not suffering from any major illness.
Then, they collected blood and urine from ill patients and healthy controls. In both cases, they then interpreted that data in two ways: by simply measuring the metabolites in the urine straight up, or doing a comparison between that metabolite and others in the same sample. This led to four separate values for both ME / CFS patients and healthy controls:
- Blood metabolites
- Absolute
- Relative
- Urine metabolites
- Absolute
- Relative
In order to understand the thrust of this article, one must keep in mind that the stuff in the urine should be 'used up', end-product-y stuff. If end products in the urine are high, that means that whatever cycle or process created them is on overdrive in the body.
It's kind of the opposite way for the blood; if something is high in the blood, it's probably not being used up very rapidly. Keep this in mind as we progress forward. If you like charts better:
View attachment 11435
Results:
Okay, guys, let's cut straight to the chase:
So, let's take a look at what this is telling us at the most basic level.
1) ME/CFS patients have hyperglycemia.
Glucose is high - both higher than in controls (absolute value) and high in comparison to other metabolites (relative value). This is saying in no uncertain terms that CFS / ME patients are hyperglycemic. It's a bold statement, actually, and ties in neatly with our neurological issues.
It also ties in with a recent study showing that, when biopsied muscle samples of ME / CFS patients are given an electric shock, the glucose in the muscle tissue does not decrease. However, that same glucose is responsive to insulin. In fact, the only reason that glucose would be in the muscle cells in any reasonable quantity is if insulin escorted it there.
This would mean that, instead of having a problem with our insulin like diabetics, we have a problem with glycolysis itself.
2) ME/CFS patients have signs of reduced glycolysis.
Look at how low pyruvate is in the urine. We are NOT flushing that stuff out; we're using it up like there's no tomorrow. That implies we're 'pushing' whatever pyruvate we DO have into the citric acid cycle. The same goes for acetate, which is oxidized pyruvate. These are the compounds that are produced 'between' glycolysis and the CAC, and the moment we get them, we're converting them to acetyl CoA and revving that Citric Acid Cycle engine as high as it'll go. The same goes for alanine and serine, which are also decreased in the urine of ME / CFS patients.
Lactate was low in blood, both relative and absolute. Shocking, you may say, given high lactate in some patients.
However, it makes sense with the reduced pyruvate. If glycolysis is scaled back, lactate, as a product of glycolysis, should be low as well. Keep in mind that this is not an exercise test, and these patients were just getting their blood drawn.
3) There are signs that other energy systems are trying to compensate.
GLUTAMATE:
The Citric Acid Cycle appears more or less intact, but a decrease in the concentrations of certain amino acids implies that they may be used to feed through the CAC, particularly glutamate, which is significantly reduced in the blood (again, showing over-utilization). Going by Hornig's two-phases hypothesis and my own experiences, however, this isn't surprising. It's quite possible that there is an excitatory initial phase of ME (with higher glutamate) and an immunosuppressive ME that presents later (with lower glutamate). (If GABA and NAC help you, high glutamate might be the culprit. If GABA and NAC made you feel weird or awful, there is a possibility that your glutamate is already low.)
Aspartate aminotransferase, which helps convert glutamate to aspartate, is elevated, as is aspartate itself. Thus, you get:
GLUTAMATE -----(Aspartate aminotransferase)---> ASPARTATE
It takes a lot of Vitamin B6 to push this reaction. Part of the reason why some of us might keel over without it could be that this is one of the compensatory energy mechanisms we're using to try to get enough without glycolysis working properly.
Low glutamate has been directly linked to diabetes and insulin resistance, although insulin resistance would appear to contradict the Newton paper showing muscle cell sensitivity to insulin.
PHENYLALANINE:
Perhaps most familiar to us because of the warning on every diet soda wrapper, phenylalanine is an amino acid that can be converted to tyrosine, which is the lead amino acid that helps us produce melatonin, thyroid hormones, serotonin, and a whole bunch of other stuff. But guess what? The reaction yields an NADP molecule, another energy molecule... so it's pushed. I'm actually a little surprised that tyrosine isn't high, but not that surprised. Given the stress the system is under in ME and CFS patients, that tyrosine may be snatched up to produce a hormone molecule the moment it appears.
CREATININE:
The creatinine being increased in the urine of ME / CFS patients implies that this reaction is being pushed. The phosphorylation of creatine isn't discussed much in Intro to Bio textbooks because it's not important in all tissues, but it is important in skeletal muscle, the heart, and the brain, where it makes ATP available super-quickly on demand - none of this three extensive, mad cycles nonsense. Think of the creatine kinase-creatinine-phosphorylcreatine system like a bank account with an ATM where you can get your energy super-quick, as opposed to investing in futures and waiting for the energy to come in (aerobic respiration!)
I've actually had this test come back a little high and been worried, because all I could find about high creatinine was kidney failure. However, it once again comes down to energy, and to us getting as much energy as we can as fast as we can grab hold of it.
This, as a mechanism, may explain why our muscles are so fatigued - we're 'borrowing' from that account with the ATM in order to pay the bills sent by our brain and heart until the muscles are bankrupt of energy, and we can't make any more withdrawals. (In fact, if we try, we may find we develop a service charge - a negative balance!)
This also may help explain why glycine is so low, because glycine makes creatine, which fuels this reaction.
Perhaps most convincingly, creatinine in the urine and glucose in the blood are directly proportional: the higher the blood sugar, the more creatinine in the ME / CFS patient's pee. This certainly implies that people who use glycolysis less need to use this pathway more.
HYPOXANTHINE and ALLANTOIN
It took me awhile to recall where I even knew this chemical from. I am very proud, because my ME-fogged-brain remembered that the answer was 'in your graduate thesis'. We had to chose an herb and outline its traditional uses, modern research, its chemistry, details of its value and how it was sold... and I, out of some puckish impulse, chose dandelion.
It didn't turn out to be a bad choice. There was oodles of information, and it was really interesting to write about something so common, but so useful.
In any case, one of the papers I read showed that dandelion flower specifically scavenged the free radicals created by converting xanthine:
View attachment 11446
This means that if hypoxanthine is reduced in the blood, then this reaction is being 'pushed'.
Weirdly, I also know allantoin through plants: it is a chemical component of comfrey (Symphytum officinale), and is considered wound-healing. It's also the product of a reaction of uric acid in certain circumstance. Since hypoxanthine is at the top of the cycle and low, it makes sense that allantoin is an end-product, and high. Once again, this reaction generates NADH, so it is an energy reaction, though I feel like I understand this one the least. Xanthine and hypoxanthine are also part of an amino acid 'scavenging' reaction which I feel is important, but I think my brain is full, now.
Since this reaction generates some serious free radicals, this may be (part of) the source of the ME / CFS patient's over-oxidation.
I recommend dandelion flower tea to mop up the peroxynitrate and hydroxy radicals. Its effect is amplified by taking it with Vitamin E, as little!me read and faithfully reported about a decade ago (thanks, little me - who knew it would come in so handy?)
Limitations of the study:
It's important to be aware that this is quite a small study that, while it suggests directions for future research, does not provide strong support for any argument. A meta-analysis is in order specifically in the case of glucose, where Armstrong et al. tell us there are several other studies that agree with their findings, but were too small to draw conclusions. A meta-analysis is when a researcher takes the data from several other studies and combines them, resulting in a larger number of subjects, and therefore more meaningful results.
Another limitation is that all of the patients came from the same physician. This unfortunately increases the chances of homogeneity amongst the participants - could they have suffered from the same pathogenic 'initial insult'? Does this doctor have a narrower image of what constitutes 'true' ME, making their issues more similar than usual, patient to patient?
Finally, it all wraps things up a bit neatly for my taste. However, it also makes a lot of sense.
Advice that Would Make Sense in the Context of this Article:
- Use antioxidant supplements
- Focus on your B-Vitamins, B-2 and B-6 especially
- Take B-2 as FMN if you can, and take HIGHER doses of CoQ-10 (not the piddling 100-200-mg) as bucket-brigade helpers for the electron transport chain
- Go Paleo, or some other diet with high healthy fats and high protein; supplement with lipases and proteases to get the best out of your digestion. Consider a protein powder.
- ...I wonder if there are amino acid multis out there. Probably. It might be a good idea to try one!
What I'm really wondering, though, is when we get to start calling it Type III Diabetes....
-Jaime