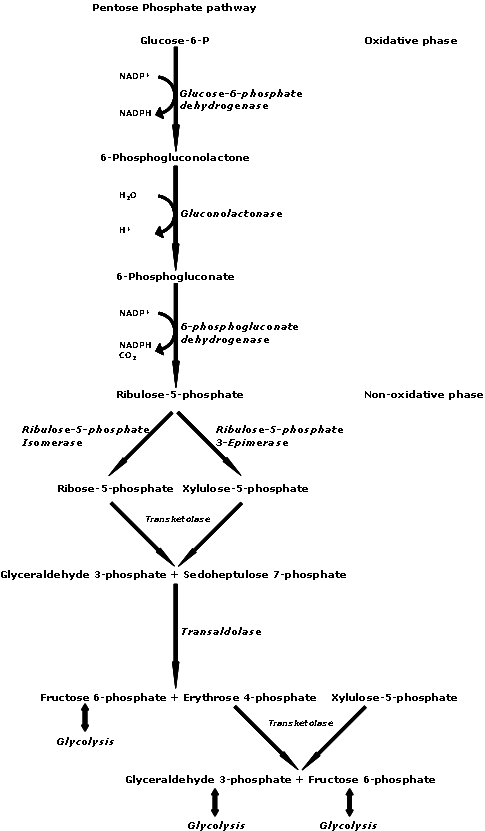
Sulfites are a big issue. If you are experiencing CFS/FMS symptoms, you should be aware of how they work and how they can affect you.
How Sulfites can Hurt You
Sulfites can hurt you if your protective Sulfite Oxidase enzyme (a chemical that converts one chemical to another chemical) is low. Sulfite Oxidase converts sulfites to sulfates, which are not
harmful. If your Sulfite Oxidase enzyme is down, the sulfites will swim around your blood and inhibit important enzymes such as Tyrosinase, polyphenoloxidase, and ascorbate oxidase. This can result in the impairment of the synthesis of Dopamine and the conversion of Dopamine to Noradrenaline, which can lead to neurological fatigue. Sulfites can inhibit 90% of lung ATP energy production, can impair liver cell ATP energy production, and can deplete glutathione (chemical that helps the liver filter the blood and helps protect cell enzymes from damage). Anything that reduces your production of ATP energy can cause fatigue, since low levels of energy are synonymous with fatigue.
Things that can inhibit your protective Sulfite Oxidase enzyme
Things that can impair the protective sulfite oxidase are as follows: heavy metal molecules such as lead and mercury, Sulfa-drugs (e.g. a class of drugs within the sulfa group that can impair pterin synthesis, such as asthmatic inhalants and many antibiotics), molybdenum deficiency, proto-IX-porphyria (enzyme that makes blood inhibited), inherited genetic damage encoding of the SO-enzyme, severe B12-vitamin deficiency, and arrays of So2/SO3-group containing drugs including DMPS (an Rx chelation drug).
How to live safely
In summary, if one wants to be safe, they should avoid things that inhibit the protective sulfite oxidase enzyme (e.g. Sulfa Drugs, heavy metals); and if this protective enzyme does go down, then avoid the Sulfites themselves.
Signs of a Sulfite problem
Below are several clues that one is sensitive to sulfites (which probably means the sulfite oxidase enzyme is down):
1) One becomes tired after ingesting one of the foods, listed above, that contains sulfites. One can look at the food label to see if the product contains sulfites.
2) One coughs after ingesting sulfites, due to the impairment of the lung ATP energy.
3) One has asthma.
4) One develops low blood glucose (sugar) after ingesting sulfites (since sulfites disrupt the regulation of blood sugar).
5) One gets a headache after ingesting sulfites. (brain fog)
6) One experiences itching and reddening of the skin after ingesting sulfites contained in foods, drinks or drugs.
Foods That Sometimes Contain Sulfites
Sulfites are a kind of food preservative. The following is a list of foods that sometimes contain sulfite preservatives: dried fruit, bottled lemon juice, bottled lime juice, red wine, molasses, sourcrout juice, grape juice (all colors), and yellow die #5 found in Doritos. Foods that sometimes contain sulfites in lesser amounts are mashed potatoes made from dry powder, pickles in a jar, shrimp, cookies, crackers, beet sugar, and pie dough. If a food contains more than 10ppm (parts per million) of sulfite, the FDA requires that the product label list the amount of sulfite. In some cases, Molybdenum, B12-vitamin, P5P-vitamin, B1-vitamin, and tetrahydrofolate supplementation has helped to boost the protective sulfite oxidase a bit. Also, if mercury or lead molecules have induced Protoporphoria (enzyme that makes blood inhibited), detoxifying those heavy metals can help as well. In some cases, the Protoporphoria is inherited, and this is considered incurable at this time. In any case, if the protective sulfite oxidase is down, one can make a great difference by avoiding sulfite containing foods.
Thiosulfate + 2 glutathione
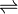 sulfite + glutathione disulfide + sulfide
sulfite + glutathione disulfide + sulfide
Thus, the two substrates of this enzyme are thiosulfate and glutathione, whereas its 3 products are sulfite, glutathione disulfide, and sulfide.
This enzyme belongs to the family of transferases, specifically the sulfurtransferases, which transfer sulfur-containing groups. The systematic name of this enzyme class is thiosulfate:thiol sulfurtransferase. Other names in common use include glutathione-dependent thiosulfate reductase, sulfane reductase, and sulfane sulfurtransferase. This enzyme participates in glutathione metabolism.
Sulfite Strategy
http://www.beatcfsandfms.org/html/Sulfides.html
Sulfite oxidase
http://en.wikipedia.org/wiki/Sulfite_oxidase
Oxidative phosphorylation
http://en.wikipedia.org/wiki/Oxidative_phosphorylation
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
http://www.ncbi.nlm.nih.gov/pubmed/16475804
Effect of sodium sulfite on mast cell degranulation and oxidant stress http://www.annallergy.org/article/S1081-1206(10)63549-1/abstract
Thiosulfate-thiol sulfurtransferase
http://en.wikipedia.org/wiki/Thiosulfate—thiol_sulfurtransferase
Molybdenum is known to function as a cofactor for four enzymes
Successful Treatment of Molybdenum Cofactor Deficiency Type A
http://pediatrics.aappublications.org/content/125/5/e1249.abstract
Molybdenm Linus Pauling Institute
http://lpi.oregonstate.edu/infocenter/minerals/molybdenum/
Functional SUOX Deficiency?
Dr. Amy Yasko, "Sulfate levels may be used as a way to assess sulfite processing. Sulfites are converted to sulfates by SUOX which uses B12 and molybdenum in the reaction. If sulfate levels are high it may indicate that sulfite levels were too high and you want to be sure that you have enough B12 and molybdenum support in place such that the levels of molybdenum and B12 are not depleted in the process of converting sulfites to sulfates. Also high sulfites (by default high sulfates) is an indication that you may have too much activity via the transulfuration pathway and so you do not yet have the methylation cycle in balance"
"There are three enzymes in the body that need molybdenum: sulfite oxidase (SUOX), xanthine oxidase, and aldehyde oxidase. Xanthine oxidase helps to convert intermediates to uric acid. Milk that has been homogenized increases the level of xanthine oxidase in the milk. This may be a more subtle aspect of dairy free diets to limit excess xanthine oxidase in the system. Too much xanthine oxidase, like increased sulfite oxidase activity runs the risk of depleting molybdenum. Finally aldehyde oxidase is involved in addressing yeast (Candida) in the body. The net effect of homogenized milk + Candida + high levels of sulfites is to increase the activity through these three molybdenum containing enzymes and potentially deplete levels of important intermediates"
"High levels of overall sulfur in the body can trigger the cortisol stress reaction. There are entire PPTs I have given on the topic of stress. Also high levels of sulfur groups in general can exacerbate other situations in the body with excess glutamate and throw off the epinephrine/norepineprhine ratio."
"One of the consequences of sulfite toxicity is the depletion of B1 vitamin in the body, B1 is also known as thiamine. Casein is one of the ways to protect vitamin B1 from destruction by sulfites, Supplementation with benfotiamine can also be considered in order to help support healthy vitamin B1 levels especially when sulfite toxicity is a concern. Tungsten in the system may impair important sulfite oxidase enzyme function. Those with higher levels of tungsten in the system may have a greater issue with sulfites and may want to support with forms of B1. In addition sulfite oxidase also needs sufficient molybdenum and B12 in the system for optimal function. So those with higher tungsten levels may also want to consider molybdenum and B12 support."
To summarize, in my opinion the issue with high sulfates is three fold, one it indicates high levels of sulfites and excess transulfuration activity, two it suggests that molybdenum and B12 may be depleted from the system and three that the cortisol reaction and imbalances in norep/epi may be a factor due to high total levels of sulfur containing groups.
Thiamine has a half-life of 18 days and is quickly exhausted, particularly when metabolic demands exceed intake. Thiamine is involved in a variety of glucose metabolism-related and neurological functions. After modification in the body to a diphosphate form, thiamine is involved in a vast array of functions:
Methionine / Methylation Chicken or the Egg
http://www.psychiatryburbankca.co/...onine_and_Methylation_Chicken_or_the_Egg1.pdf
No Sulfites Help for Food Allergy
http://www.learningtarget.com/nosulfites/
Foods high in sulfur (thiols)
http://www.livingnetwork.co.za/chelationnetwork/food/high-sulfur-sulphur-food-list/
Methionine synthase catalyzes the final step in the regeneration of methionine from homocysteine. The overall reaction transforms 5-methyltetrahydrofolateinto tetrahydrofolate (THF) while transferring a methyl group to Hcy to form Met. Methionine synthase is the only mammalian enzyme that metabolizes N5-MeTHF to regenerate the active cofactor THF. In cobalamin-dependent forms of the enzyme, the reaction proceeds by two steps in a ping-pong reaction. The enzyme is initially primed into a reactive state by the transfer of a methyl group from N5-MeTHF to Co(I) in enzyme-bound cobalamin, forming methyl-cobalamin that now contains Me-Co(III) and activating the enzyme. Then, a Hcy that has coordinated to an enzyme-bound zinc to form a reactive thiolate reacts with the Me-Cob. The activated methyl group is transferred from Me-Cob to the Hcy thiolate, which regenerates Co(I) in cob, and Met is released from the enzyme. The cob-independent mechanism follows the same general pathway but with a direct reaction between the zinc thiolate and N5-MeTHF.
The mechanism of the enzyme depends on the constant regeneration of Co(I) in cob, but this is not always guaranteed. Instead, every 1-2000 catalytic turnovers, the Co(I) may be oxidized into Co(II), which would permanently shut down catalytic activity. A separate protein, Methionine Synthase Reductase, catalyzes the regeneration of Co(II) and the restoration of enzymatic activity. Because this process inevitably shuts down all methionine synthase activity, defects or deficiencies in methionine synthase reductase have been implicated in some of the disease associations for methionine synthase deficiency.
Cysteine and cystine
As the functional group of the amino acid cysteine, the thiol group plays a very important role in biology. When the thiol groups of two cysteine residues (as in monomers or constituent units) are brought near each other in the course of protein folding, an oxidation reaction can generate a cystine unit with a disulfide bond (-S-S-). Disulfide bonds can contribute to a protein's tertiary structure if the cysteines are part of the same peptide chain, or contribute to the quaternary structure of multi-unit proteins by forming fairly strong covalent bonds between different peptide chains. A physical manifestation of cysteine-cystine equilibrium is provided by hair straightening technologies.
Sulfhydryl groups in the active site of an enzyme can form noncovalent bonds with the enzyme's substrate as well, contributing to covalent catalytic activity in catalytic triads. Active site cysteine residues are the functional unit in cysteine protease catalytic triads. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning.
Mark Hyman, MD
http://experiencelife.com/article/functional-wellness-part-6-energy-mitochondria-and-toxicity/
Cofactors
Many cofactors (non-protein-based helper molecules) feature thiols. The biosynthesis and degradation of fatty acids and related long-chain hydrocarbons is conducted on a scaffold that anchors the growing chain through a thioester derived from the thiol Coenzyme A. The biosynthesis of methane, the principal hydrocarbon on earth, arises from the reaction mediated by coenzyme M, 2-mercaptoethyl sulfonic acid. Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, especially those classified as soft. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Molecular Aspects of Thimerosal-induced Autism
http://www.whale.to/a/deth.pdf
Phosphatidylinositide 3-kinases
http://en.wikipedia.org/wiki/Phosphoinositide_3-kinase
Dr. Richard Deth
Methionine synthase
http://en.wikipedia.org/wiki/Methionine_synthase
Thiol
http://en.wikipedia.org/wiki/Thiol
Pantothenic acid
http://en.wikipedia.org/wiki/Pantothenate
How Sulfites can Hurt You
Sulfites can hurt you if your protective Sulfite Oxidase enzyme (a chemical that converts one chemical to another chemical) is low. Sulfite Oxidase converts sulfites to sulfates, which are not
harmful. If your Sulfite Oxidase enzyme is down, the sulfites will swim around your blood and inhibit important enzymes such as Tyrosinase, polyphenoloxidase, and ascorbate oxidase. This can result in the impairment of the synthesis of Dopamine and the conversion of Dopamine to Noradrenaline, which can lead to neurological fatigue. Sulfites can inhibit 90% of lung ATP energy production, can impair liver cell ATP energy production, and can deplete glutathione (chemical that helps the liver filter the blood and helps protect cell enzymes from damage). Anything that reduces your production of ATP energy can cause fatigue, since low levels of energy are synonymous with fatigue.
Things that can inhibit your protective Sulfite Oxidase enzyme
Things that can impair the protective sulfite oxidase are as follows: heavy metal molecules such as lead and mercury, Sulfa-drugs (e.g. a class of drugs within the sulfa group that can impair pterin synthesis, such as asthmatic inhalants and many antibiotics), molybdenum deficiency, proto-IX-porphyria (enzyme that makes blood inhibited), inherited genetic damage encoding of the SO-enzyme, severe B12-vitamin deficiency, and arrays of So2/SO3-group containing drugs including DMPS (an Rx chelation drug).
How to live safely
In summary, if one wants to be safe, they should avoid things that inhibit the protective sulfite oxidase enzyme (e.g. Sulfa Drugs, heavy metals); and if this protective enzyme does go down, then avoid the Sulfites themselves.
Signs of a Sulfite problem
Below are several clues that one is sensitive to sulfites (which probably means the sulfite oxidase enzyme is down):
1) One becomes tired after ingesting one of the foods, listed above, that contains sulfites. One can look at the food label to see if the product contains sulfites.
2) One coughs after ingesting sulfites, due to the impairment of the lung ATP energy.
3) One has asthma.
4) One develops low blood glucose (sugar) after ingesting sulfites (since sulfites disrupt the regulation of blood sugar).
5) One gets a headache after ingesting sulfites. (brain fog)
6) One experiences itching and reddening of the skin after ingesting sulfites contained in foods, drinks or drugs.
Foods That Sometimes Contain Sulfites
Sulfites are a kind of food preservative. The following is a list of foods that sometimes contain sulfite preservatives: dried fruit, bottled lemon juice, bottled lime juice, red wine, molasses, sourcrout juice, grape juice (all colors), and yellow die #5 found in Doritos. Foods that sometimes contain sulfites in lesser amounts are mashed potatoes made from dry powder, pickles in a jar, shrimp, cookies, crackers, beet sugar, and pie dough. If a food contains more than 10ppm (parts per million) of sulfite, the FDA requires that the product label list the amount of sulfite. In some cases, Molybdenum, B12-vitamin, P5P-vitamin, B1-vitamin, and tetrahydrofolate supplementation has helped to boost the protective sulfite oxidase a bit. Also, if mercury or lead molecules have induced Protoporphoria (enzyme that makes blood inhibited), detoxifying those heavy metals can help as well. In some cases, the Protoporphoria is inherited, and this is considered incurable at this time. In any case, if the protective sulfite oxidase is down, one can make a great difference by avoiding sulfite containing foods.
Thiosulfate + 2 glutathione
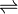
Thus, the two substrates of this enzyme are thiosulfate and glutathione, whereas its 3 products are sulfite, glutathione disulfide, and sulfide.
This enzyme belongs to the family of transferases, specifically the sulfurtransferases, which transfer sulfur-containing groups. The systematic name of this enzyme class is thiosulfate:thiol sulfurtransferase. Other names in common use include glutathione-dependent thiosulfate reductase, sulfane reductase, and sulfane sulfurtransferase. This enzyme participates in glutathione metabolism.
Sulfite Strategy
http://www.beatcfsandfms.org/html/Sulfides.html
Sulfite oxidase
http://en.wikipedia.org/wiki/Sulfite_oxidase
Oxidative phosphorylation
http://en.wikipedia.org/wiki/Oxidative_phosphorylation
The G473D mutation impairs dimerization and catalysis in human sulfite oxidase
http://www.ncbi.nlm.nih.gov/pubmed/16475804
Effect of sodium sulfite on mast cell degranulation and oxidant stress http://www.annallergy.org/article/S1081-1206(10)63549-1/abstract
Thiosulfate-thiol sulfurtransferase
http://en.wikipedia.org/wiki/Thiosulfate—thiol_sulfurtransferase
Molybdenum is known to function as a cofactor for four enzymes
- Sulfite oxidase catalyzes the transformation of sulfite to sulfate, a reaction that is necessary for the metabolism of sulfur-containing amino acids (methionine and cysteine).
- Xanthine oxidase catalyzes the breakdown of nucleotides (precursors to DNA and RNA) to form uric acid, which contributes to the plasma antioxidant capacity of the blood.
- Aldehyde oxidase and xanthine oxidase catalyze hydroxylation reactions that involve a number of different molecules with similar chemical structures. Xanthine oxidase and aldehyde oxidase also play a role in the metabolism of drugs and toxins.
- Mitochondrial amidoxime reducing component (mARC) was described only recently (4), and its precise function is under investigation. Initial studies showed that mARC forms a three-component enzyme system with cytochrome b5 and NADH cytochrome b5 reductase that catalyzes the detoxification of mutagenic N-hydroxylated bases.
Successful Treatment of Molybdenum Cofactor Deficiency Type A
http://pediatrics.aappublications.org/content/125/5/e1249.abstract
Molybdenm Linus Pauling Institute
http://lpi.oregonstate.edu/infocenter/minerals/molybdenum/
Functional SUOX Deficiency?
Dr. Amy Yasko, "Sulfate levels may be used as a way to assess sulfite processing. Sulfites are converted to sulfates by SUOX which uses B12 and molybdenum in the reaction. If sulfate levels are high it may indicate that sulfite levels were too high and you want to be sure that you have enough B12 and molybdenum support in place such that the levels of molybdenum and B12 are not depleted in the process of converting sulfites to sulfates. Also high sulfites (by default high sulfates) is an indication that you may have too much activity via the transulfuration pathway and so you do not yet have the methylation cycle in balance"
"There are three enzymes in the body that need molybdenum: sulfite oxidase (SUOX), xanthine oxidase, and aldehyde oxidase. Xanthine oxidase helps to convert intermediates to uric acid. Milk that has been homogenized increases the level of xanthine oxidase in the milk. This may be a more subtle aspect of dairy free diets to limit excess xanthine oxidase in the system. Too much xanthine oxidase, like increased sulfite oxidase activity runs the risk of depleting molybdenum. Finally aldehyde oxidase is involved in addressing yeast (Candida) in the body. The net effect of homogenized milk + Candida + high levels of sulfites is to increase the activity through these three molybdenum containing enzymes and potentially deplete levels of important intermediates"
"High levels of overall sulfur in the body can trigger the cortisol stress reaction. There are entire PPTs I have given on the topic of stress. Also high levels of sulfur groups in general can exacerbate other situations in the body with excess glutamate and throw off the epinephrine/norepineprhine ratio."
"One of the consequences of sulfite toxicity is the depletion of B1 vitamin in the body, B1 is also known as thiamine. Casein is one of the ways to protect vitamin B1 from destruction by sulfites, Supplementation with benfotiamine can also be considered in order to help support healthy vitamin B1 levels especially when sulfite toxicity is a concern. Tungsten in the system may impair important sulfite oxidase enzyme function. Those with higher levels of tungsten in the system may have a greater issue with sulfites and may want to support with forms of B1. In addition sulfite oxidase also needs sufficient molybdenum and B12 in the system for optimal function. So those with higher tungsten levels may also want to consider molybdenum and B12 support."
To summarize, in my opinion the issue with high sulfates is three fold, one it indicates high levels of sulfites and excess transulfuration activity, two it suggests that molybdenum and B12 may be depleted from the system and three that the cortisol reaction and imbalances in norep/epi may be a factor due to high total levels of sulfur containing groups.
Thiamine has a half-life of 18 days and is quickly exhausted, particularly when metabolic demands exceed intake. Thiamine is involved in a variety of glucose metabolism-related and neurological functions. After modification in the body to a diphosphate form, thiamine is involved in a vast array of functions:
- Carbohydrate metabolism
- Production of the neurotransmitters glutamic acid and GABA, through the TCA cycle
- Lipid metabolism, necessary for myelin production
- Amino acid metabolism
- Neuromodulation.
Methionine / Methylation Chicken or the Egg
http://www.psychiatryburbankca.co/...onine_and_Methylation_Chicken_or_the_Egg1.pdf
No Sulfites Help for Food Allergy
http://www.learningtarget.com/nosulfites/
Foods high in sulfur (thiols)
http://www.livingnetwork.co.za/chelationnetwork/food/high-sulfur-sulphur-food-list/
Methionine synthase catalyzes the final step in the regeneration of methionine from homocysteine. The overall reaction transforms 5-methyltetrahydrofolateinto tetrahydrofolate (THF) while transferring a methyl group to Hcy to form Met. Methionine synthase is the only mammalian enzyme that metabolizes N5-MeTHF to regenerate the active cofactor THF. In cobalamin-dependent forms of the enzyme, the reaction proceeds by two steps in a ping-pong reaction. The enzyme is initially primed into a reactive state by the transfer of a methyl group from N5-MeTHF to Co(I) in enzyme-bound cobalamin, forming methyl-cobalamin that now contains Me-Co(III) and activating the enzyme. Then, a Hcy that has coordinated to an enzyme-bound zinc to form a reactive thiolate reacts with the Me-Cob. The activated methyl group is transferred from Me-Cob to the Hcy thiolate, which regenerates Co(I) in cob, and Met is released from the enzyme. The cob-independent mechanism follows the same general pathway but with a direct reaction between the zinc thiolate and N5-MeTHF.
The mechanism of the enzyme depends on the constant regeneration of Co(I) in cob, but this is not always guaranteed. Instead, every 1-2000 catalytic turnovers, the Co(I) may be oxidized into Co(II), which would permanently shut down catalytic activity. A separate protein, Methionine Synthase Reductase, catalyzes the regeneration of Co(II) and the restoration of enzymatic activity. Because this process inevitably shuts down all methionine synthase activity, defects or deficiencies in methionine synthase reductase have been implicated in some of the disease associations for methionine synthase deficiency.
Cysteine and cystine
As the functional group of the amino acid cysteine, the thiol group plays a very important role in biology. When the thiol groups of two cysteine residues (as in monomers or constituent units) are brought near each other in the course of protein folding, an oxidation reaction can generate a cystine unit with a disulfide bond (-S-S-). Disulfide bonds can contribute to a protein's tertiary structure if the cysteines are part of the same peptide chain, or contribute to the quaternary structure of multi-unit proteins by forming fairly strong covalent bonds between different peptide chains. A physical manifestation of cysteine-cystine equilibrium is provided by hair straightening technologies.
Sulfhydryl groups in the active site of an enzyme can form noncovalent bonds with the enzyme's substrate as well, contributing to covalent catalytic activity in catalytic triads. Active site cysteine residues are the functional unit in cysteine protease catalytic triads. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning.
Mark Hyman, MD
http://experiencelife.com/article/functional-wellness-part-6-energy-mitochondria-and-toxicity/
Cofactors
Many cofactors (non-protein-based helper molecules) feature thiols. The biosynthesis and degradation of fatty acids and related long-chain hydrocarbons is conducted on a scaffold that anchors the growing chain through a thioester derived from the thiol Coenzyme A. The biosynthesis of methane, the principal hydrocarbon on earth, arises from the reaction mediated by coenzyme M, 2-mercaptoethyl sulfonic acid. Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, especially those classified as soft. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Molecular Aspects of Thimerosal-induced Autism
http://www.whale.to/a/deth.pdf
Phosphatidylinositide 3-kinases
http://en.wikipedia.org/wiki/Phosphoinositide_3-kinase
Dr. Richard Deth
Methionine synthase
http://en.wikipedia.org/wiki/Methionine_synthase
Thiol
http://en.wikipedia.org/wiki/Thiol
Pantothenic acid
http://en.wikipedia.org/wiki/Pantothenate
Last edited: