Tom Kindlon
Senior Member
- Messages
- 1,734
-------------------------Dear Mr. Smallcombe,
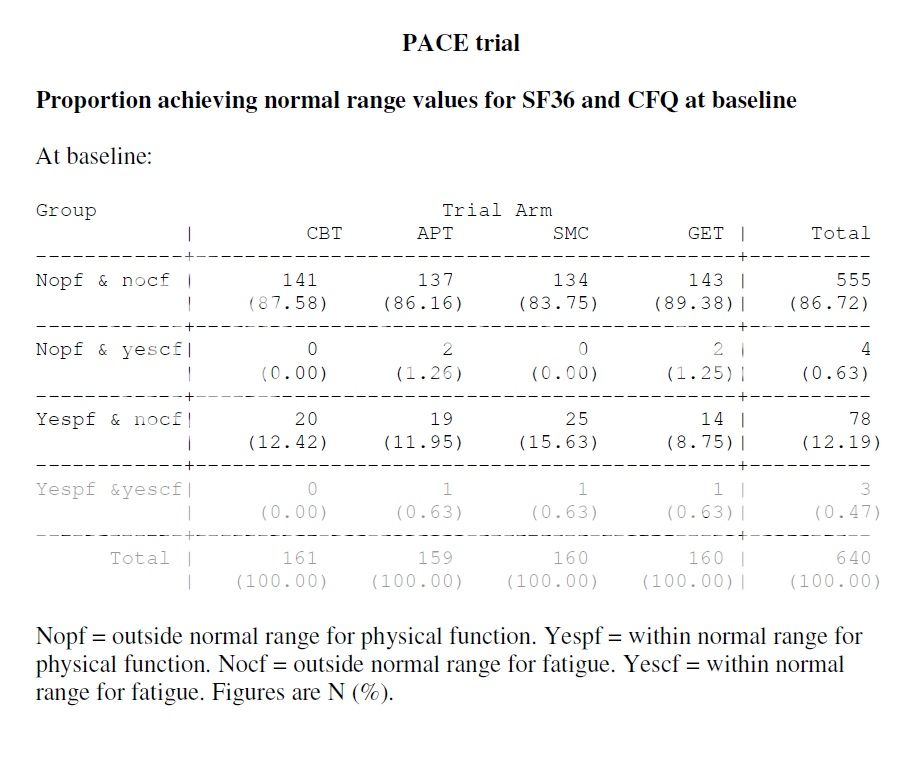
Thank you very much for your reply. It appears that I did not ask my question well enough however. Would it be possible to ask how many participants were in the normal range in either the SF-36 or Chalder Fatigue Scale at the beginning of the trial, ie one or the other?
Thank you very much for your time.
Regards,
<name>
(Queen Mary, University of London) FOI Request: 2013/F42
On Mon, Mar 11, 2013, <foi-enquiries@qmul.ac.uk> wrote:
Dear <name>
Thank you for your email of 12th February.
Please see the attached for the information you requested.
If you are dissatisfied with this response, you may ask the College to conduct a review of this decision. To do this, please contact the College in writing (including by fax, letter or email), describe the original request, explain your grounds for dissatisfaction, and include an address for correspondence. You have 40 working days from receipt of this communication to submit a review request. When the review process has been completed, if you are still dissatisfied, you may ask the Information Commissioner to intervene. Please see www.ico.gov.uk for details.
Yours sincerely
Paul Smallcombe
Records & Information Compliance Manager