RogerBlack
Senior Member
- Messages
- 902
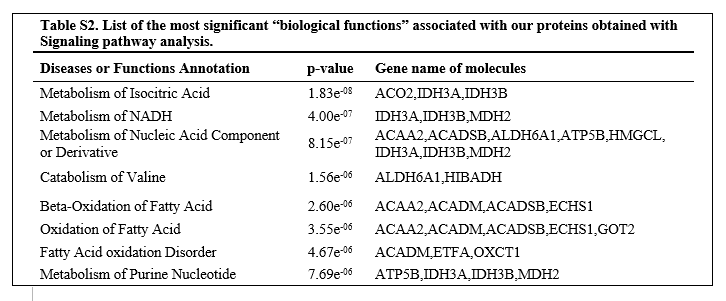
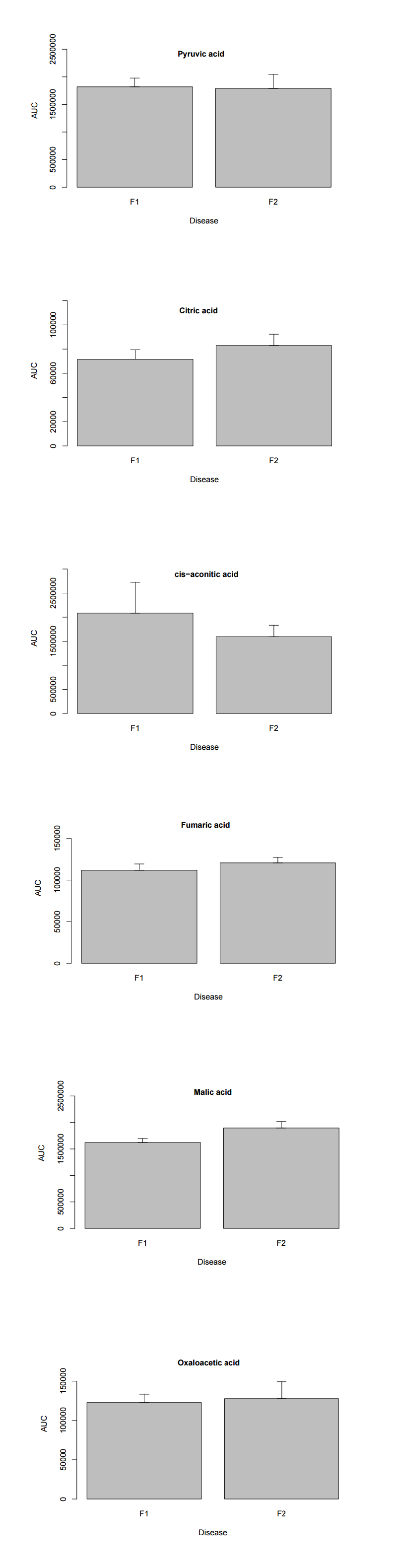
In these kinds of systems, it's sort of like a mass production facility. Think of the protein as a machine in a factory. Putting a label on a bottle for example. If you had enough labels, enough power, enough bottles, enough labour, enough space, enough waste management, and efficient transportation for your labels and bottles to get from and to your machine,... Than sure, twice as fast.
But as this is different in CFS, it is likely various of the above is not true, and though more protein has been put in to do a task, it may not even reach the normal efficiency.
There are a few scenarios.
The machinery has correctly determined that the rate of what the protein is doing is too low, and what it's doing in adding it helps, though does not fix the situation.
The machinery does not directly regulate the level of protein, and it's a symptom of something else going wrong, but doesn't do much.
Something is triggering activation of the protein, when it should not be, and its increased level is hurting.
Working out if the increased level of any protein 'means' something clinically significant is hard, and requires deep understanding of the biochemistry and what is the limiting factor. Even quite large changes in some levels may be clinically insignificant.
Last edited: