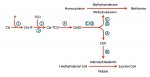
I know there are many people who advocate the use of both methyl b12 and methyl folate use simultaneously for the purpose of moving the folate cycle through the methylate homocysteine.
As far as my understanding goes, 5-methyl THF donates methyl to cobalamin, which becomes methyl cobalamin, and then that methyl is transferred to homocysteine to form methionine.
According to many places on this forum, methyl trapping can occur when one is deficient of b12, so 5-mTFH cannot pass its methyl group to cobalamin and be converted back into THF.
The reason I bring this up is because I was just reading this article about autism in which it states,
"Methyl-B12: Replaces need for methyl group transfer from 5methylTHF: potential for trapping folate as 5-methyl- THF and reducing synthesis of metabolically active THF".
Article: (http://www.tacanow.org/wp-content/uploads/2010/07/glutathione-autism-1.pdf)
In this way it almost sounds as if too much methylb12 will inhibit the passing of methyl from folate because it's already methylated causing trapping. Thoughts on this?
What the heck is going on inside of a normal person?? What form of cobalamin normally exists in this reaction? It can't be methyl cobalamin. Methyl cobalamin is the RESULT of methyl transfer from 5 mTFH.
In this textbook "Postgraduate Haemotology" it states that 5- deoxyadenosyl (ado) is the main form of cobalamin in nature and in human tissue". Is this the same as the "adenosyl" form that many of you refer to?
source:
(http://books.google.com/booksid=1A6...6AEwBTgU#v=onepage&q=glutathionyl b12&f=false)
As far as my understanding goes, 5-methyl THF donates methyl to cobalamin, which becomes methyl cobalamin, and then that methyl is transferred to homocysteine to form methionine.
According to many places on this forum, methyl trapping can occur when one is deficient of b12, so 5-mTFH cannot pass its methyl group to cobalamin and be converted back into THF.
The reason I bring this up is because I was just reading this article about autism in which it states,
"Methyl-B12: Replaces need for methyl group transfer from 5methylTHF: potential for trapping folate as 5-methyl- THF and reducing synthesis of metabolically active THF".
Article: (http://www.tacanow.org/wp-content/uploads/2010/07/glutathione-autism-1.pdf)
In this way it almost sounds as if too much methylb12 will inhibit the passing of methyl from folate because it's already methylated causing trapping. Thoughts on this?
What the heck is going on inside of a normal person?? What form of cobalamin normally exists in this reaction? It can't be methyl cobalamin. Methyl cobalamin is the RESULT of methyl transfer from 5 mTFH.
In this textbook "Postgraduate Haemotology" it states that 5- deoxyadenosyl (ado) is the main form of cobalamin in nature and in human tissue". Is this the same as the "adenosyl" form that many of you refer to?
source:
(http://books.google.com/booksid=1A6...6AEwBTgU#v=onepage&q=glutathionyl b12&f=false)