Sasha submitted a new blog post:
Fluge & Mella's pre-trial study highlights life-changing potential of rituximab
Sasha gives the background and Simon gives the interpretation of the latest study from Haukeland, published today...
It’s out! Dr Øystein Fluge and Professor Olav Mella have published their new study in PLoS ONE. And though the study was not a blinded, placebo-controlled trial, the results are further evidence that rituximab is beneficial in some ME/CFS patients, and potentially life-changing for a substantial minority. The findings also give important new insights into the optimum dosing schedule to maintain those benefits in the long run.
We all know the story that led up to this study: cancer specialists Fluge and Mella treated an ME patient for cancer with the immune-system drug rituximab. The patient’s ME symptoms improved markedly. The effect was confirmed in two additional patients and so Fluge and Mella set up a 30-patient, double-blind, randomised, placebo-controlled trial of rituximab in ME/CFS.
The results, when published in October 2011, were striking: although there were no differences between the two groups at the pre-defined endpoint of three months, 67% of patients receiving rituximab had moderate to major improvement during follow-up, compared to only 13% of placebo patients (p=0.003).
And not only that: there was a tell-tale delay of several months in the improvement. This time-lag suggested that it was rituximab’s B-cell destroying capabilities that were responsible. That, in turn suggested that rituximab was switching off an autoimmune process (some B-cells produce antibodies that attack the body’s own tissues).
The findings took the ME/CFS world by storm and started a sea-change in attitudes to the disease. The Norwegian Health Directorate apologised for how ME patients had been treated. Researchers and clinicians outside the ME/CFS community began to focus on autoimmunity as a hypothesis rather than psychology. Fundraising efforts for more rituximab trials started up all over the world, including MEandYou’s successful crowdfund in Norway and the ongoing fundraising for Invest in ME’s UK rituximab trial and B-cell studies.
Meanwhile, Fluge and Mella set up a small study – this newly published study – to inform the design of a larger trial. In their original placebo-controlled study, most of the patients who benefited from rituximab had a transient response and then relapsed. Fluge and Mella needed to investigate the best dosing schedule, and other aspects of rituximab’s use.
The large multi-centre rituximab trial is now underway in Norway, led by Fluge and Mella and funded by MEandYou, the Norwegian government, and others. It’s another double-blind, placebo-controlled, randomised study and has 152 patients, who will be followed up for 24 months post-treatment: results won’t be available until 2017 or 2018.
Meanwhile, this new study has much to tell us. Over to Simon, to take a look at it.
The science
Thanks, Sasha...
The study findings are very encouraging. 64% of patients showed a clinical response, similar to the 67% seen in the original trial. Half were classed as major responders. And at the end of the study a quarter of patients were doing exceptionally well: in my opinion the data suggests they were close to recovery.
Most of these patients had featured in the earlier trials: nine from the trial placebo group, nine from the rituximab group and one pilot patient.
Only ten patients were new, so response rates for this and the previous trial are largely based on a small and highly overlapping group of patients. The response rates seen here might not be replicated in a wider group of patients (leaving aside that this and the earlier trial are small studies).
A word of caution
I want to point out the limitations of this study – limitations the authors note in their paper – before I enthuse about how good some of these results seem to me.
The goal was to help establish the therapeutic effect of repeated maintenance doses of rituximab on long-term response, and to help guide the design of the large clinical trial now under way in Norway. However, it wasn’t a gold-standard placebo-controlled randomised trial, and wasn’t meant to be, and that means we have to be careful about interpreting the results.
First, the study was unblinded – patients and clinicians knew they were dealing with a drug with great potential. That can artificially boost results, to a modest degree.
Second, there was no control group (ideally a placebo group to see how patients would have responded to a dummy treatment), and some of the observed gains might have occurred even if no drug had been given.
And finally, 20 out of the 29 patients had been in previous rituximab studies.
More details are in the box.
How the study worked
All patients met both Fukuda and Canadian consensus criteria. The average age of patients was 40, and 69% were women. They’d been ill for an average of nine years, and 20 (two-thirds) were at least housebound, including three who were mainly bedbound.
The main measure of success was change in fatigue from baseline (see box below). For clinical response, patients needed six consecutive weeks of improvement, and to average at least a slight-to-moderate improvement, including at least one week better than moderate (so at least one of the fatigue symptoms had to be rated as a major improvement). This could be at any time during the 36 months of the study.
Patients received an initial two doses of rituximab two weeks apart and then maintenance infusions at 3, 6, 10 and 15 months. They were followed up for a total 36 months.
How fatigue was measured
Results
Of the 28 patients who received any maintenance doses, 18 (64%) showed a clinical response, similar to the 67% seen in the initial trial. 14 were classed as major responders and four as ‘moderate responders’.
Thus, half of the patients showed a ‘major’ response, with an average response period of 105 weeks (out of 156 weeks in the study). Typically, patients didn’t start improving until 23 weeks after the first dose of rituximab.
Peak response, and relapse for some
The results for patients overall peaked at 20 to 24 months, which was 5 to 9 months after most patients had had their final dose. (Some patients who were responding slowly received more, but these additional doses didn’t seem to make much difference.)
During this peak response time, the average SF-36 scores for major responders matched average population scores (though some were well below average, at levels typically seen in people who have long-term illness). That’s a big improvement from baseline when the scores showed they were all well and truly sick.
However, some patients declined after this peak period, including four major responders who, after doing extremely well, relapsed severely – almost back to baseline levels.
Even so, nine out of 14 major responders (32% of patients) were still showing a clinical response, of various levels, at the end of the study. Including moderate responders, eleven were still in response at the end of the study, showing that repeated rituximab dosing had given a sustained response, right to the end of the study for some.
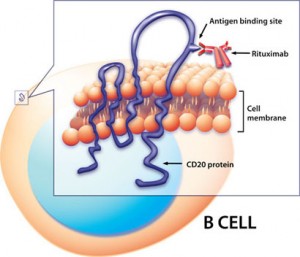 Rituximab in action: binds to CD20 protein on the B-cell surface, triggering cell death. Credit: NIAID
Rituximab in action: binds to CD20 protein on the B-cell surface, triggering cell death. Credit: NIAID
Mega-responders?
However, for me, the big story of this study is the substantial group who did exceptionally well, though I should stress this is my interpretation of the data in the paper rather than anything the authors have claimed. There is a wealth of data in the paper, down to the level of individual patients. Seven patients – a quarter of those who had maintenance rituximab doses – showed a response that looks close to recovery at the end of the trial, that is, at 32 to 36 months, which is the final data point on the graph of outcomes. Fatigue, SF-36 Physical Function and self-rated daily functioning scores all look very impressive:
While the placebo effect and response-bias may occur, they are relatively modest effects. And with ME/CFS, natural recovery rates are low. So these ‘mega-responder’ results strike me as very impressive, and important. Such life-changing improvements are not a common feature of ME/CFS clinical trials.
In the paper, the authors say:
“Some have now had no symptoms for five years," added Fluge, talking to the New Scientist, which also quoted Nancy Klimas:
Safety
How does this compare with the PACE Trial?
Just to put them in some sort of context, it's worth making a rough comparison (given available data) with the PACE Trial, whose cognitive behavioural therapy (CBT) and graded exercise therapy (GET) are often presented as the best treatments currently available to ME/CFS patients.
Obviously, PACE was a randomised trial, was very large, and had a control group – but there was no placebo group and the study was 'unblinded' like this one: that is, patients knew if they were in an active therapy group.
The PACE Trial authors claimed a 22% 'recovery' rate for both CBT and GET (compared with 8% for controls), but the recovery rate was built on the primary outcomes of fatigue and function, including an SF-36 Physical Function score of just 60, compared with the 85 I used above. These recovery criteria have been criticised as too weak, both by patients and at least one proponent of CBT.
On this basis, with a quarter scoring 85 or over on SF36 Physical Function, the new rituximab results look very good compared with PACE. We will, of course, have to wait until the full randomised controlled rituximab trial that is under way in Norway is complete before a proper comparison is possible. But in the meantime I think these results are very promising indeed.
Do not try this at home!
These results are promising but please bear in mind the authors’ caution: “We do not encourage the use of rituximab for ME/CFS outside of approved clinical trials, and this is especially important for the group with very severe disease.”
They also reported trying rituximab on four very severely affected patients, with disappointing results.
Evidence of Autoimmunithy
The authors finish up by pointing out that their new results are further evidence that a subgroup of ME/CFS patients have an autoimmune disease. Rituximab is a proven treatment for several autoimmune diseases. It destroys B-cells, wiping out the supply of autoantibodies. Antibodies are produced in bulk by plasma cells (created from B-cells). These are not affected by rituximab and so continue to produce antibodies for several months before dying off. That could explain the pronounced time-lag seen in the clinical responses: it’s not until plasma cells die off and are not replaced (because B-cells have been wiped out) that autoantibodies decline and symptoms improve.
However, say the authors, other mechanisms than autoimmunity may explain the observed clinical effects of B-cell depletion on ME/CFS symptom maintenance. Rituximab influences other types of immune function, including T-cell functioning.
Winning over sceptics
These impressive new results for rituximab are already having an impact. Professor Sir Simon Wessely, a critic of the original rituximab trial, is impressed by the latest findings: “There is now a strong case to be made for a larger trial”, he told the New Scientist. Happily, just such a trial is in progress.
Disclaimer: my section of the blog was put together in a hurry. The paper is huge and while I think I’ve given a fair (though by no means comprehensive) report of the study there may be errors. If so, please let me know!
And back to Sasha...
An opportunity!
This story may well hit the news and if it does, we should hijack the media with our own messages: that ME/CFS is a serious, organic disease and that people should donate to the biomedical research charities (and in this instance, it would be particularly appropriate to mention Invest in ME’s UK rituximab trial fund). This is a great opportunity to educate, win hearts and minds, and grow our donor base. This recent article explains how.
And of course, we too can donate, including via JustGiving. Let’s get to it!
Further resources
Compilation of Phoenix Rising tweets on Dr Fluge and Professor Mella's rituximab presentation at the 2015 Invest in ME conference
Dr Ros Vallings's summary of the 2015 Invest in ME conference, including an account of Dr Fluge and Professor Mella's rituximab presentation
Simon McGrath blogs about ME/CFS research
There are many ways you can help Phoenix Rising to continue its work. If you feel able to offer your time and talent, we could really use some more authors, proof-readers, fundraisers, technicians etc. We’d also love to expand our Board of Directors. So, if you think you can help in any way then please contact Mark through the Forums.
And don’t forget: you can always support our efforts at no cost to yourself as you shop online! To find out more, visit Phoenix Rising’s Donate page by clicking the button below.
Continue reading the Original Blog Post
Fluge & Mella's pre-trial study highlights life-changing potential of rituximab
Sasha gives the background and Simon gives the interpretation of the latest study from Haukeland, published today...
It’s out! Dr Øystein Fluge and Professor Olav Mella have published their new study in PLoS ONE. And though the study was not a blinded, placebo-controlled trial, the results are further evidence that rituximab is beneficial in some ME/CFS patients, and potentially life-changing for a substantial minority. The findings also give important new insights into the optimum dosing schedule to maintain those benefits in the long run.
We all know the story that led up to this study: cancer specialists Fluge and Mella treated an ME patient for cancer with the immune-system drug rituximab. The patient’s ME symptoms improved markedly. The effect was confirmed in two additional patients and so Fluge and Mella set up a 30-patient, double-blind, randomised, placebo-controlled trial of rituximab in ME/CFS.
The results, when published in October 2011, were striking: although there were no differences between the two groups at the pre-defined endpoint of three months, 67% of patients receiving rituximab had moderate to major improvement during follow-up, compared to only 13% of placebo patients (p=0.003).
And not only that: there was a tell-tale delay of several months in the improvement. This time-lag suggested that it was rituximab’s B-cell destroying capabilities that were responsible. That, in turn suggested that rituximab was switching off an autoimmune process (some B-cells produce antibodies that attack the body’s own tissues).
The findings took the ME/CFS world by storm and started a sea-change in attitudes to the disease. The Norwegian Health Directorate apologised for how ME patients had been treated. Researchers and clinicians outside the ME/CFS community began to focus on autoimmunity as a hypothesis rather than psychology. Fundraising efforts for more rituximab trials started up all over the world, including MEandYou’s successful crowdfund in Norway and the ongoing fundraising for Invest in ME’s UK rituximab trial and B-cell studies.
Meanwhile, Fluge and Mella set up a small study – this newly published study – to inform the design of a larger trial. In their original placebo-controlled study, most of the patients who benefited from rituximab had a transient response and then relapsed. Fluge and Mella needed to investigate the best dosing schedule, and other aspects of rituximab’s use.
The large multi-centre rituximab trial is now underway in Norway, led by Fluge and Mella and funded by MEandYou, the Norwegian government, and others. It’s another double-blind, placebo-controlled, randomised study and has 152 patients, who will be followed up for 24 months post-treatment: results won’t be available until 2017 or 2018.
Meanwhile, this new study has much to tell us. Over to Simon, to take a look at it.
The science
Thanks, Sasha...
In a subgroup of ME/CFS patients, prolonged B-cell depletion with rituximab maintenance infusions was associated with sustained clinical responses.
Dr Fluge, Professor Mella and colleagues
Dr Fluge, Professor Mella and colleagues
The study findings are very encouraging. 64% of patients showed a clinical response, similar to the 67% seen in the original trial. Half were classed as major responders. And at the end of the study a quarter of patients were doing exceptionally well: in my opinion the data suggests they were close to recovery.
Some limitations
This was an ‘open label’ trial, in which all patients and medical staff knew the patients were on rituximab, and there was no control group. Some of the improvements recorded might have been down to placebo response, and/or ‘response bias’, in which patients subconsciously overstate their improvements (all outcomes were self-reported). Some patients may have simply improved naturally, regardless of the rituximab treatment.
Most of these patients had featured in the earlier trials: nine from the trial placebo group, nine from the rituximab group and one pilot patient.
Only ten patients were new, so response rates for this and the previous trial are largely based on a small and highly overlapping group of patients. The response rates seen here might not be replicated in a wider group of patients (leaving aside that this and the earlier trial are small studies).
The only way to remove such problems is to conduct a randomised, double-blind, placebo-controlled trial – which is exactly what Fluge and Mella have organised, with a large multi-centre trial under way in Norway. This latest study was set up to help plan that large trial..
A word of caution
I want to point out the limitations of this study – limitations the authors note in their paper – before I enthuse about how good some of these results seem to me.
The goal was to help establish the therapeutic effect of repeated maintenance doses of rituximab on long-term response, and to help guide the design of the large clinical trial now under way in Norway. However, it wasn’t a gold-standard placebo-controlled randomised trial, and wasn’t meant to be, and that means we have to be careful about interpreting the results.
First, the study was unblinded – patients and clinicians knew they were dealing with a drug with great potential. That can artificially boost results, to a modest degree.
Second, there was no control group (ideally a placebo group to see how patients would have responded to a dummy treatment), and some of the observed gains might have occurred even if no drug had been given.
And finally, 20 out of the 29 patients had been in previous rituximab studies.
More details are in the box.
How the study worked
All patients met both Fukuda and Canadian consensus criteria. The average age of patients was 40, and 69% were women. They’d been ill for an average of nine years, and 20 (two-thirds) were at least housebound, including three who were mainly bedbound.
The main measure of success was change in fatigue from baseline (see box below). For clinical response, patients needed six consecutive weeks of improvement, and to average at least a slight-to-moderate improvement, including at least one week better than moderate (so at least one of the fatigue symptoms had to be rated as a major improvement). This could be at any time during the 36 months of the study.
Patients received an initial two doses of rituximab two weeks apart and then maintenance infusions at 3, 6, 10 and 15 months. They were followed up for a total 36 months.
How fatigue was measured
Patients scored four different aspects of fatigue (fatigue, malaise after exertion, need for rest, and lack of daily functioning) on a scale of 0 to 6. The overall fatigue score was the average of the scores for the four different aspects.
On the 0-6 scale, 3 was the midpoint, meaning there was no difference from baseline. 4, 5 and 6 corresponded to slight, moderate and major improvement from baseline, respectively. 2, 1 and 0 meant slight, moderate and major worsening, respectively.
This was the same scale as used in the small placebo-controlled trial. Although this scale hasn't been formally validated, the authors also used the validated and widely used SF-36 health scale, and the SF-36 results told the same story as the fatigue scale.
Results
"Eleven of the 18 responders were still in remission three years after beginning the treatment, and some have now had no symptoms for five years," says Fluge. "Suddenly, their limbs started to work again and their hands were no longer cold or sweaty." (From an interview in the New Scientist)
Of the 28 patients who received any maintenance doses, 18 (64%) showed a clinical response, similar to the 67% seen in the initial trial. 14 were classed as major responders and four as ‘moderate responders’.
Thus, half of the patients showed a ‘major’ response, with an average response period of 105 weeks (out of 156 weeks in the study). Typically, patients didn’t start improving until 23 weeks after the first dose of rituximab.
Peak response, and relapse for some
The results for patients overall peaked at 20 to 24 months, which was 5 to 9 months after most patients had had their final dose. (Some patients who were responding slowly received more, but these additional doses didn’t seem to make much difference.)
During this peak response time, the average SF-36 scores for major responders matched average population scores (though some were well below average, at levels typically seen in people who have long-term illness). That’s a big improvement from baseline when the scores showed they were all well and truly sick.
However, some patients declined after this peak period, including four major responders who, after doing extremely well, relapsed severely – almost back to baseline levels.
Even so, nine out of 14 major responders (32% of patients) were still showing a clinical response, of various levels, at the end of the study. Including moderate responders, eleven were still in response at the end of the study, showing that repeated rituximab dosing had given a sustained response, right to the end of the study for some.
 Rituximab in action: binds to CD20 protein on the B-cell surface, triggering cell death. Credit: NIAID
Rituximab in action: binds to CD20 protein on the B-cell surface, triggering cell death. Credit: NIAIDMega-responders?
However, for me, the big story of this study is the substantial group who did exceptionally well, though I should stress this is my interpretation of the data in the paper rather than anything the authors have claimed. There is a wealth of data in the paper, down to the level of individual patients. Seven patients – a quarter of those who had maintenance rituximab doses – showed a response that looks close to recovery at the end of the trial, that is, at 32 to 36 months, which is the final data point on the graph of outcomes. Fatigue, SF-36 Physical Function and self-rated daily functioning scores all look very impressive:
- Seven patients reported the maximum possible fatigue improvement from baseline, that is, major improvement in all four fatigue symptoms. One patient was actually just shy of the maximum, scoring approximately 5.9 out of 6.0 (Fig 2A).
- Seven had a SF-36 Physical Function score of 85 or more, which is equal to or better than the population average (Fig 5A).
- Seven had function levels of 80% or higher (someone at 80%-90% is defined as having "slight restrictions in physical or social functioning, who may perform all activities almost as a completely healthy person, but at a reduced pace or duration"), with two scoring 100% (Fig 6B).
While the placebo effect and response-bias may occur, they are relatively modest effects. And with ME/CFS, natural recovery rates are low. So these ‘mega-responder’ results strike me as very impressive, and important. Such life-changing improvements are not a common feature of ME/CFS clinical trials.
In the paper, the authors say:
"One pilot patient is still in complete response with no ME/CFS symptoms even after vigorous exercise, five years after the first, and 3 ½ years after the last rituximab infusion”.
“Some have now had no symptoms for five years," added Fluge, talking to the New Scientist, which also quoted Nancy Klimas:
"I am very intrigued by the rituximab story," says Nancy Klimas, an authority on CFS at Nova Southeastern University in Fort Lauderdale, Florida. "It's particularly exciting when people seem to have experienced very long periods of remission, and even speak of recovery," she says.
Safety
“There was no unexpected toxicity.”
One patient had an allergic reaction to the initial rituximab dose and took no further part in the trial, leaving 28 patients who passed the ‘induction’ phase. A second had an allergic reaction at the three-month infusion and received no further rituximab doses.
Two patients had an episode of uncomplicated late-onset neutropenia, with recovery after five days. ('Neutropenia' means low levels of neutrophils, which are first-response immune cells that are in the front line of the body’s immune response. Late-onset neutropenia is a known side-effect of rituximab treatment: 9% of patients on rituximab got it in one study, but neutrophil levels normally recover.)
Eight patients experienced one or more transient ME/CFS symptom flares after rituximab infusions. One patient developed breast cancer but this was not judged to be related to rituximab.
As Fluge and Mella state:
Two patients had an episode of uncomplicated late-onset neutropenia, with recovery after five days. ('Neutropenia' means low levels of neutrophils, which are first-response immune cells that are in the front line of the body’s immune response. Late-onset neutropenia is a known side-effect of rituximab treatment: 9% of patients on rituximab got it in one study, but neutrophil levels normally recover.)
Eight patients experienced one or more transient ME/CFS symptom flares after rituximab infusions. One patient developed breast cancer but this was not judged to be related to rituximab.
As Fluge and Mella state:
“Rituximab maintenance treatment is generally considered safe [the]. However, even if severe adverse effects are rare, they may occur and include defects in immune reconstitution, and reactivation of chronic viral infections such as hepatitis."
Safety for ME/CFS will be assessed further in the ongoing large clinical trial. Monitoring of rituximab in other illnesses indicate it is generally safe, but not without some risk.
Just to put them in some sort of context, it's worth making a rough comparison (given available data) with the PACE Trial, whose cognitive behavioural therapy (CBT) and graded exercise therapy (GET) are often presented as the best treatments currently available to ME/CFS patients.
Obviously, PACE was a randomised trial, was very large, and had a control group – but there was no placebo group and the study was 'unblinded' like this one: that is, patients knew if they were in an active therapy group.
The PACE Trial authors claimed a 22% 'recovery' rate for both CBT and GET (compared with 8% for controls), but the recovery rate was built on the primary outcomes of fatigue and function, including an SF-36 Physical Function score of just 60, compared with the 85 I used above. These recovery criteria have been criticised as too weak, both by patients and at least one proponent of CBT.
On this basis, with a quarter scoring 85 or over on SF36 Physical Function, the new rituximab results look very good compared with PACE. We will, of course, have to wait until the full randomised controlled rituximab trial that is under way in Norway is complete before a proper comparison is possible. But in the meantime I think these results are very promising indeed.
Do not try this at home!
These results are promising but please bear in mind the authors’ caution: “We do not encourage the use of rituximab for ME/CFS outside of approved clinical trials, and this is especially important for the group with very severe disease.”
They also reported trying rituximab on four very severely affected patients, with disappointing results.
Evidence of Autoimmunithy
The authors finish up by pointing out that their new results are further evidence that a subgroup of ME/CFS patients have an autoimmune disease. Rituximab is a proven treatment for several autoimmune diseases. It destroys B-cells, wiping out the supply of autoantibodies. Antibodies are produced in bulk by plasma cells (created from B-cells). These are not affected by rituximab and so continue to produce antibodies for several months before dying off. That could explain the pronounced time-lag seen in the clinical responses: it’s not until plasma cells die off and are not replaced (because B-cells have been wiped out) that autoantibodies decline and symptoms improve.
However, say the authors, other mechanisms than autoimmunity may explain the observed clinical effects of B-cell depletion on ME/CFS symptom maintenance. Rituximab influences other types of immune function, including T-cell functioning.
Winning over sceptics
These impressive new results for rituximab are already having an impact. Professor Sir Simon Wessely, a critic of the original rituximab trial, is impressed by the latest findings: “There is now a strong case to be made for a larger trial”, he told the New Scientist. Happily, just such a trial is in progress.
Disclaimer: my section of the blog was put together in a hurry. The paper is huge and while I think I’ve given a fair (though by no means comprehensive) report of the study there may be errors. If so, please let me know!
And back to Sasha...
An opportunity!
This story may well hit the news and if it does, we should hijack the media with our own messages: that ME/CFS is a serious, organic disease and that people should donate to the biomedical research charities (and in this instance, it would be particularly appropriate to mention Invest in ME’s UK rituximab trial fund). This is a great opportunity to educate, win hearts and minds, and grow our donor base. This recent article explains how.
And of course, we too can donate, including via JustGiving. Let’s get to it!
Further resources
Compilation of Phoenix Rising tweets on Dr Fluge and Professor Mella's rituximab presentation at the 2015 Invest in ME conference
Dr Ros Vallings's summary of the 2015 Invest in ME conference, including an account of Dr Fluge and Professor Mella's rituximab presentation
Simon McGrath blogs about ME/CFS research
There are many ways you can help Phoenix Rising to continue its work. If you feel able to offer your time and talent, we could really use some more authors, proof-readers, fundraisers, technicians etc. We’d also love to expand our Board of Directors. So, if you think you can help in any way then please contact Mark through the Forums.
And don’t forget: you can always support our efforts at no cost to yourself as you shop online! To find out more, visit Phoenix Rising’s Donate page by clicking the button below.
Continue reading the Original Blog Post
Last edited by a moderator:





