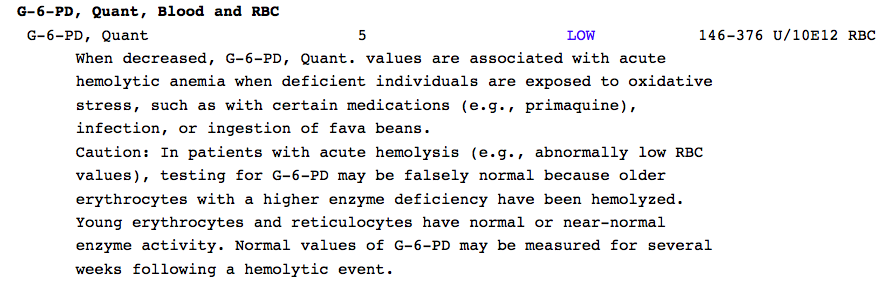
G6PDD
Glucose-6-phosphate dehydrogenase deficiency (several studies published about polymorfisms from Mediterranean, African and SE Asian continents)
≠
Glucose-6-phosphate dehydrogenase allele deletion (I am having a hard time in finding studies on this)
My husband carries a G6PD allele deletion and is 99.9% of northern European descent, also has downstream deleterious SNPs, but neither he or his siblings ever had anemia or jaundice, so any health complication they might have will never be associated with G6PDD.
My husband was a sports enthusiast in his teens and early 20's, but after some infections he dropped sports and since 2004 suffers from extreme PEM.
https://www.sciencedirect.com/science/article/pii/S0006497120617190
Volume 136, Issue 11, 10 September 2020, Pages 1225-1240
Journal home page for Blood
Inherited Anemias
Glucose-6-phosphate dehydrogenase deficiency
Authors from Tanzania and Italy
Abstract
Glucose 6-phosphate dehydrogenase (G6PD) deficiency is 1 of the commonest human enzymopathies, caused by inherited mutations of the X-linked gene G6PD. G6PD deficiency makes red cells highly vulnerable to oxidative damage, and therefore susceptible to hemolysis. Over 200 G6PD mutations are known: approximately one-half are polymorphic and therefore common in various populations. Some 500 million persons with any of these mutations are mostly asymptomatic throughout their lifetime; however, any of them may develop acute and sometimes very severe hemolytic anemia when triggered by ingestion of fava beans, by any of a number of drugs (for example, primaquine, rasburicase), or, more rarely, by infection. Approximately one-half of the G6PD mutations are instead sporadic: rare patients with these mutations present with chronic nonspherocytic hemolytic anemia. Almost all G6PD mutations are missense mutations, causing amino acid replacements that entail deficiency of G6PD enzyme activity: they compromise the stability of the protein, the catalytic activity is decreased, or a combination of both mechanisms occurs. Thus, genotype-phenotype correlations have been reasonably well clarified in many cases. G6PD deficiency correlates remarkably, in its geographic distribution, with past/present malaria endemicity: indeed, it is a unique example of an X-linked human polymorphism balanced through protection of heterozygotes from malaria mortality. Acute hemolytic anemia can be managed effectively provided it is promptly diagnosed. Reliable diagnostic procedures are available, with point-of-care tests becoming increasingly important where primaquine and its recently introduced analog tafenoquine are required for the elimination of malaria.

(C) Time course of Hb levels in a large cohort of children with P falciparum malaria who received antimalarial treatment with a drug combination (Lapdap) containing dapsone. Eleven percent of G6PD-deficient hemizygous boys and 0.5% of heterozygous girls required blood transfusion. All of these children were in clinical trials under appropriate medical supervision; there were no deaths (there might have been outside of clinical trials).
AHA = acute hemolytic anemia