My first reaction: probably not, because T cells are too few in comparison to the other cells in the body to have a large impact on metabolomics.
However they seem to be reporting exactly that.
You have to realize that a great deal of signalling involves cascades of signalling cells and molecules. I'm guessing that cytotoxic T-cells are near the beginning, because killing target cells has powerful and long-lasting effects, an extreme kind of amplification. I'm also thinking that the changes in use of amino acids within cells will have significant effects on the cascade of signals involved in purinergic signalling.
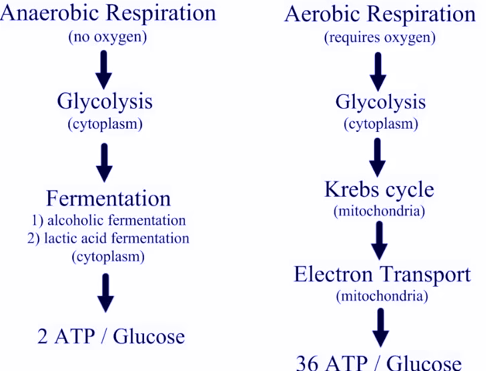
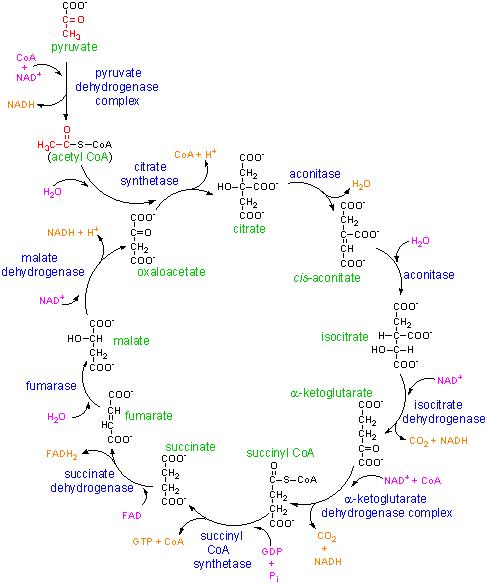
We tend to think of molecules like ATP as simply fuel produced by mitochondria, and consumed by other organelles. In fact these molecules also have very powerful actions in signalling activity of nearby cells, triggering changes that would not take place simply because there was a metabolic disturbance elsewhere in the body. We are seeing evidence here that specific subpopulations of immune cells are undergoing big changes in activity. These should result in substantial changes in the signals going to other cells near them.
Take a look at the paper I linked in
another post, about the gut microbiome and autoimmunity. In that case we see cytotoxic T-cells destroying dendritic cells in the gut to suppress inflammatory response in the gut. This is not all that unusual for immune cells. Different populations of immune cells fight it out to achieve a specific response while limiting damage from autoimmunity. When this fails you get damage like type 1 diabetes or unsuppressed inflammation in the gut, two things you would not normally consider related.
Dendritic cells are near the root of this signalling cascade, even though their textbook description as merely "antigen-presenting cells" makes them sound like minor passive components of immune response.
We now know they are much more active, e.g. picking up information on pathogens from maternal cells when an infant's naive immune system needs a quick-start guide to a new environment. Once they have acquired antigens in the gut, they relocate to lymph nodes, where they pass the information to both B-cells and T-cells. I'm still a little vague on how they behave in the pancreas and other organs, but I'm assuming this is also a very active operation.
We have also learned that they keep antigens in a separate compartment, allowing actual HIV virions in maternal milk or immune cells to infect other immune cells even though the dendritic cell, which carried the virion, itself remains uninfected.
Signals near the beginning of a cascade are necessarily weak and hard to find, because they have not yet been amplified by other cells in the immune system. A great deal of our understanding will change as research continues, but we are finally close to the root of many pathological processes.