- Messages
- 88
I'm not sure how much in detail this has been discussed but I'd like to have a discussion about Mark Davis' findings that he revealed at the Stanford ME/CFS symposium, on Saturday.
Here is the direct link:

As an initial disclaimer, I am the furthest thing from an expert in immunology. I've been trying to learn about it over the past few days in order to make sense of Mark's findings.
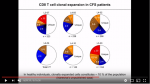
Essentially, he found clonal expansion of CD8+ (cytotoxic) T-cells in ME/CFS patients. If I understand it correctly, the body will clone these cytotoxic T-cells when it needs to build an army to fight an antigen. This also happens to patients that have other diseases like cancer, MS, lyme disease, and probably many more. Mark Davis did say that it is possible these cytotoxic T-cells are evidence that ME/CFS is an autoimmune disease and that these cytotoxic T-cells may be targeting the self; hence autoimmunity. He mentioned the microglia as a possible errand target for the cytotoxic T-cells.
Here are some thoughts. Whatever is happening with these T-cells must make sense within the context of how some patients respond and recover from ME/CFS when given Rituximab and/or cyclophosphamide (the CycloME study hasn't been released but according to the researchers there are many responders, and cyclo is "at least" as promising as Rituximab). Could the large number of cytotoxic T-cells be evidence of an ongoing viral infection? I doubt it, since if it were an ongoing viral infection the Rituximab would wipe it out very quickly, instead of a long lag-time (according to Fluge and Mella). It seems like the clonal expansion of cytotoxic T-cells could have been initially caused by infections; since ME/CFS is often caused by an initial infection. Then the cells either hang around because they got confused and found a new self target by mistake, or the cytotoxic T-cells didn't decline back to healthy levels (around 10% of total T-cells according to Dr. Davis, if I am understanding that correctly) for some unknown reason. Could the success of cyclo be in that it reduces the number of T-cells, thus killing cytotoxic cells and re-starting the T-cell population? I believe cylco has an immunostimulatory effect in low dosages and an immunosuppresive effect in higher dosages. Below is a paper showing that 100mg of cyclo per day can significantly decrease T and B cell counts.
http://onlinelibrary.wiley.com/doi/10.1002/art.1780180113/pdf
Is the 1 to 1.5g of cyclo given every 4 weeks (in the CycloME trial) enough to also significantly decrease T and B cell counts? It seems like it, otherwise, there wouldn't be massive risks of infections caused by the therapy. Could the response, by some patients, be due to this reset of T and B cell populations? Rituximab only effects B-cells but B-cells and T-cells do work together so Rituximab would still impact the function of T-cells, I think?
Does anyone else have any thoughts? Am I completely misunderstanding this stuff?
Cheers,
Cam
Here is the direct link:
As an initial disclaimer, I am the furthest thing from an expert in immunology. I've been trying to learn about it over the past few days in order to make sense of Mark's findings.
Essentially, he found clonal expansion of CD8+ (cytotoxic) T-cells in ME/CFS patients. If I understand it correctly, the body will clone these cytotoxic T-cells when it needs to build an army to fight an antigen. This also happens to patients that have other diseases like cancer, MS, lyme disease, and probably many more. Mark Davis did say that it is possible these cytotoxic T-cells are evidence that ME/CFS is an autoimmune disease and that these cytotoxic T-cells may be targeting the self; hence autoimmunity. He mentioned the microglia as a possible errand target for the cytotoxic T-cells.
Here are some thoughts. Whatever is happening with these T-cells must make sense within the context of how some patients respond and recover from ME/CFS when given Rituximab and/or cyclophosphamide (the CycloME study hasn't been released but according to the researchers there are many responders, and cyclo is "at least" as promising as Rituximab). Could the large number of cytotoxic T-cells be evidence of an ongoing viral infection? I doubt it, since if it were an ongoing viral infection the Rituximab would wipe it out very quickly, instead of a long lag-time (according to Fluge and Mella). It seems like the clonal expansion of cytotoxic T-cells could have been initially caused by infections; since ME/CFS is often caused by an initial infection. Then the cells either hang around because they got confused and found a new self target by mistake, or the cytotoxic T-cells didn't decline back to healthy levels (around 10% of total T-cells according to Dr. Davis, if I am understanding that correctly) for some unknown reason. Could the success of cyclo be in that it reduces the number of T-cells, thus killing cytotoxic cells and re-starting the T-cell population? I believe cylco has an immunostimulatory effect in low dosages and an immunosuppresive effect in higher dosages. Below is a paper showing that 100mg of cyclo per day can significantly decrease T and B cell counts.
http://onlinelibrary.wiley.com/doi/10.1002/art.1780180113/pdf
Is the 1 to 1.5g of cyclo given every 4 weeks (in the CycloME trial) enough to also significantly decrease T and B cell counts? It seems like it, otherwise, there wouldn't be massive risks of infections caused by the therapy. Could the response, by some patients, be due to this reset of T and B cell populations? Rituximab only effects B-cells but B-cells and T-cells do work together so Rituximab would still impact the function of T-cells, I think?
Does anyone else have any thoughts? Am I completely misunderstanding this stuff?
Cheers,
Cam