The ICD-10 CM codes are online.
ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD10CM/2015/
Download the pdf and look under the Tabular Listing.
The link above is no longer the most recent release of ICD-10-CM (which is updated annually).
The most recent release is this one:
https://www.cdc.gov/nchs/icd/icd10cm.htm#FY 2018 release of ICD-10-CM
2018 release of ICD-10-CM
The 2018 ICD-10-CM codes are to be used from October 1, 2017 through September 30, 2018.
Note: This replaces the FY 2017 release.
The PDF for the FY 2018 Release can be downloaded from this page from Zip files:
ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD10CM/2018/
But for ease of access, the 2018 Tabular List and Index can be downloaded as a PDFs from my
Dx Revision Watch site:
Tabular List FY 2018:
https://dxrevisionwatch.files.wordpress.com/2017/09/icd10cm_tabular_2018.pdf
Index FY 2018:
https://dxrevisionwatch.files.wordpress.com/2017/09/icd10cm_index_2018.pdf
It is correct that no definitions, descriptions or guidance for coders and other end users is included for any of the three terms within the WHO's unmodified ICD-10, the US specific ICD-10-CM or other country modifications, for example, Canada's ICD-10-CA, Australia's ICD-10-AM and Germany's ICD-10-GM.
You might see on blogs and websites reference to the "WHO's definition of ME" with a textual description. This is a misconception - there is no "WHO definition" of ME or CFS and the text that often accompanies this misconception is an extract from the Canadian Guidelines - not from any WHO statement or document.
WHO, Geneva has never set out in a public statement or document what it understands by the terms "PVFS", "(Benign) ME" and "CFS" or how it views the relationship between these three terms.
For the WHO's ICD-10, the three terms continue to be coded (and in the case of CFS, indexed) at G93.3, in the
Diseases of the nervous system chapter.
For ICD-11 Beta draft, all three terms are currently listed under the parent class:
Other disorders of the nervous system. BME and CFS are both included in the ICD-11 equivalent of the Tabular List and are specified as inclusion terms under
Title concept, PVFS.
For ICD-11 Beta draft, exclusions have been added for BME and CFS under "Fatigue" ("Malaise and fatigue" in ICD-10) in the ICD-11 Symptoms, signs chapter.
For a history of the evolving coding of PVFS, ME and CFS in ICD-9, ICD-9-CM, ICD-10 and ICD-10-CM to March 2001, see the CDC archived document:
A Summary of Chronic Fatigue Syndrome and Its Classification in the International Classification of Diseases
Prepared by the Centers for Disease Control and Prevention, National Center for Health Statistics, Office of the Center Director, Data Policy and Standards,
CDC archive document, March 2001.
The development of an ICD-10-CM/PCS based on the WHO's ICD-10, was a very long time in preparation.
The draft of the Tabular List of ICD-10-CM was made available on the NCHS website for public comment from December 1997 through February 1998. There were several iterations before a draft was finally implemented in October 2015 - 23 years after the WHO had finalized and disseminated ICD-10.
By 2001, the point at which the document above was published, the proposal for the three terms had been to locate all three terms under G93.3:
Extract:
"ICD-10-CM
In keeping with the placement in the ICD-10, chronic fatigue syndrome (and its synonymous terms) will remain at G93.3 in ICD-10-CM.
"While it appears most appropriate to classify chronic fatigue syndrome in ICD-10 CM in the same way that it is classified in ICD-10, this placement is not without problems. The primary concern with the current WHO placement in ICD-10 has been that the abnormalities of the brain in chronic fatigue syndrome patients most often cited in the literature are not found in all chronic fatigue syndrome patients. While chronic fatigue syndrome may be a heterogeneous group of disorders, some but not all are neurological in nature. Likewise, not all patients have experienced a viral infection prior to being diagnosed with chronic fatigue syndrome, nor are immune system anomalies universally found. Also of potential concern is the similarity between the type of neurological findings in chronic fatigue syndrome and in depression, which is a psychiatric disorder. Involvement of multiple systems has complicated the classification of chronic fatigue syndrome."
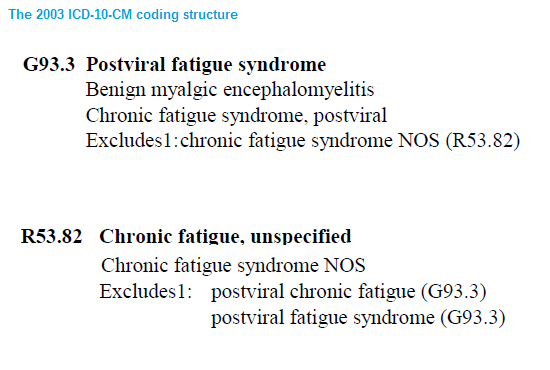
This is how the draft ICD-10-CM had stood in the draft Tabular List, as released in
2003.
There had been a "Chronic fatigue syndrome, postviral" included under G93.3 and a "Chronic fatigue syndrome NOS (Not Otherwise Specified)" as an inclusion under
R52.82 Chronic fatigue, unspecified, in the
Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified (R00-R99) chapter.
The R code chapter
"includes symptoms, signs, abnormal results of clinical or other investigative procedures, and ill defined conditions regarding which no diagnosis classifiable elsewhere is recorded."
By
2004, it became apparent (via a presentation given by William Reeves at a CFSAC meeting attended by Mary Schweitzer) that a change of structure from the 2003 proposals had been effected.
"Chronic fatigue syndrome, postviral" had been removed from G93.3.
There was no revised draft released between 2003 and 2006 but when a 2007 draft Tabular List was posted, this is how the revised structure now stood and how it currently stands:
So between the 2003 draft iteration and the 2007 draft iteration, the inclusion term, "Chronic fatigue syndrome, postviral" under G93.3 had been deleted.
Which left the only listing for "Chronic fatigue syndrome" the listing under the R codes, as: "Chronic fatigue syndrome NOS" (Not Otherwise Specified).
CDC's Donna Pickett has stated (at a CFSAC presentation and at an NCHS/CDC ICD-10-CM Coordination and Maintenance Committee meeting presentation) that where there is evidence of viral onset, the clinician/coder can use the G93.3 code. Where there is insufficient evidence of viral onset, the R53.82 code can be used.
According to a background document Dr Wanda Jones presented to a May 2011 CFSAC Committee meeting:
"As it relates to CFS the use of two codes is consistent with the classification as there would be a code to capture CFS when the physician has determined the cause as being due to a past viral infection (G93.3) or if the physician has not established a link with a past viral infection (R53.82).
"If code R53.82 were eliminated it would not be possible to disaggregate cases that are now distinguishable through the use of two codes.
"There is a general equivalence map between ICD-9-CM and ICD-10-CM codes, however, if a concept is not carried over from the earlier version to the newer version data will be lost going forward."
Source: Extract: ICD-related questions from CFSAC for May 2011 meeting
Dr. Jones clarified for the Committee that if, in the clinician's judgment, it was considered there is enough evidence to attribute the patient's illness to a viral illness onset then the clinician could code to G93.3 (Postviral fatigue syndrome). If
"however they could not identify where the trajectory developed toward CFS, then it would wind up in the R codes."
It has been further confirmed that testing for a viral illness is not required to assign a code – that coding is based on the clinician's judgment.
And from the NCHS September 14, 2011 Coordination and Maintenance Committee meeting
Proposals document:
"In ICD-10-CM chronic fatigue syndrome NOS (that is not specified as being due to a past viral infection) was added to ICD-10-CM in Chapter 18 at R53.82, Chronic fatigue, unspecified. ICD-10-CM retained code G93.3 to allow the differentiation of cases of fatigue syndrome where the physician has determined the cause as being due to a past viral infection from cases where the physician has not established a post viral link. It should be noted that including chronic fatigue syndrome NOS at code G93.3 would make it difficult to disaggregate cases that are now distinguishable through the use of two separate codes."
Hope this historical material may be of interest.