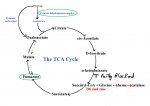
I have revised my theory again, so I will try to share it in an understandable way. I am calling it the Glutathione Depletion Cascade, because I think that it starts when a person has stress, often from more than one source, that overcomes the body's ability to produce enough glutatione (and catalase) to deal with the hydrogen peroxide, or H2O2, produced by oxidative stress. H2O2 levels build up and affect other enzymes by interfering with iron-sulfur clusters (used in the electron transport chain to make energy, and for other things, too) and TCA cycle (energy cycle) enzymes and sulfite oxidase isn't able to get rid of sulfite and that builds up and causes more problems and a whole cascade of enzyme inhibition is started which creates a vicious cycle which continues even if the original stress goes away.
This all leads to disruptions in the energy producing cycle and low levels of catalase and norepinephrine, and high H2O2 and sulfite and a lot of messed up metabolic pathways.
We have figured out some very helpful ways of treating it, but not how to overcome the vicious cycle and put an end to it. I think it could be stopped if there was a way to lower the sulfite levels, but I can't find anything to do that yet.
I am working on a blog to explain all this so if anyone is interested, it is here. I have graphics that I hope will help people to understand what I am taking about, plus a Powerpoint about it.
I really think this is probably the main cause of ME/CFS, and a number of other chronic illnesses involving mitochondrial dysfunction. Some of my ideas about treatment are different than what are usually talked about, such as high dose niacinamide (essential), and an alternative source of acetyl groups for the TCA cycle (but not ketones!), particularly in the brain. (also essential)
This all leads to disruptions in the energy producing cycle and low levels of catalase and norepinephrine, and high H2O2 and sulfite and a lot of messed up metabolic pathways.
We have figured out some very helpful ways of treating it, but not how to overcome the vicious cycle and put an end to it. I think it could be stopped if there was a way to lower the sulfite levels, but I can't find anything to do that yet.
I am working on a blog to explain all this so if anyone is interested, it is here. I have graphics that I hope will help people to understand what I am taking about, plus a Powerpoint about it.
I really think this is probably the main cause of ME/CFS, and a number of other chronic illnesses involving mitochondrial dysfunction. Some of my ideas about treatment are different than what are usually talked about, such as high dose niacinamide (essential), and an alternative source of acetyl groups for the TCA cycle (but not ketones!), particularly in the brain. (also essential)